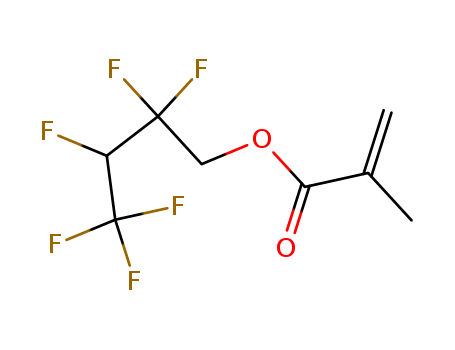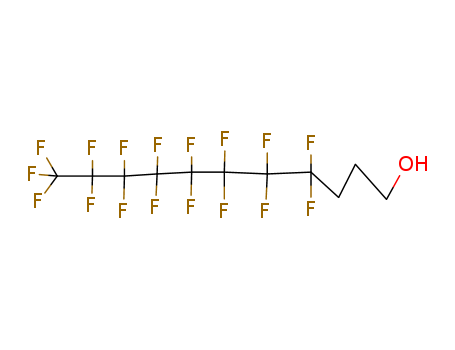
1651-41-8
- Product Name:3-(Perfluorooctyl)Propanol
- MF:C11H7 F17 O
- Purity:99%
- Molecular Weight:478.149
Product Details
Cost-effective customized wholesale 3-(Perfluorooctyl)Propanol 1651-41-8
- Molecular Formula:C11H7 F17 O
- Molecular Weight:478.149
- Melting Point:42 °C
- Boiling Point:196.7°Cat760mmHg
- PKA:14.84±0.10(Predicted)
- Flash Point:72.8°C
- PSA:20.23000
- Density:1.59g/cm3
- LogP:5.76830
1651-41-8 Relevant articles
Synthesis and antimicrobial activity of a perfluoroalkyl-containing quaternary ammonium salt
Shao, Hui,Jiang, Li,Meng, Wei-Dong,Qing, Feng-Ling
, p. 89 - 91 (2003)
A novel perfluoroalkyl-containing quater...
Synthesis and surface antimicrobial activity of a novel perfluorooctylated quaternary ammonium silane coupling agent
Shao, Hui,Meng, Wei-Dong,Qing, Feng-Ling
, p. 721 - 724 (2004)
A novel perfluorooctyl-containing quater...
Fluorous biphasic catalysis. 2. Synthesis of fluoroponytailed amine ligands along with fluoroponytailed carboxylate synthons, [M(C8F17(CH2)2CO2) 2] (M = Mn2+ or Co2+)
Vincent,Rabion,Yachandra,Fish
, p. 888 - 895 (2001)
Fluorous biphasic catalysis (FBC) is a r...
A convenient access to triarylphosphines with fluorous phase affinity
Sinou, Denis,Pozzi, Gianluca,Hope, Eric G.,Stuart, Alison M.
, p. 849 - 852 (1999)
Perfluorocarbon-soluble triarylphosphine...
Copper(I) complexes mediated cyclization reaction of unsaturated ester under fluoro biphasic procedure
De Campo, Floryan,Lastecoueres, Dominique,Vincent, Jean-Marc,Verlhac, Jean-Baptiste
, p. 4969 - 4971 (1999)
-
Synthesis of 3-perfluoroalkyl-propyl esters of L-(+)-tartaric acid
Szlavik, Zoltan,Tarkanyi, Gabor,Tarczay, Gyoergy,Goemoery, Agnes,Rabai, Jozsef
, p. 83 - 87 (1999)
A convenient and effective method for th...
Syntheses of fluorous propenes from 3-perfluoroalkyl-2-iodo-1-propanols
Szíjjártó, Csongor,Ivanko, Peter,Takács, Ferenc T.,Szabó, Dénes,Rábai, József
, p. 386 - 389 (2008)
3-(Perfluoroalkyl)-1-propenes are obtain...
Self-assembled monolayers on gold generated from terminally perfluorinated alkanethiols bearing propyl vs. ethyl hydrocarbon spacers
Zenasni, Oussama,Jamison, Andrew C.,Marquez, Maria D.,Lee, T. Randall
, p. 128 - 136 (2015/03/05)
This paper examines the structural and i...
Mesophase structure of low-wetting liquid crystalline polyacrylates with new perfluoroalkyl benzoate side groups
Martinelli, Elisa,Paoli, Francesca,Gallot, Bernard,Galli, Giancarlo
experimental part, p. 4128 - 4139 (2011/10/30)
The synthesis, thermal behavior, bulk mi...
Br?nsted acidic imidazolium salts containing perfluoroalkyl tails catalyzed one-pot synthesis of 1,8-dioxo-decahydroacridines in water
Shen, Wei,Wang, Li-Min,Tian, He,Tang, Jun,Yu, Jian-jun
body text, p. 522 - 527 (2009/11/30)
One-pot three-component synthesis of 1,8...
Towards oligosaccharide library synthesis by fluorous mixture method
Tojino, Mami,Mizuno, Mamoru
scheme or table, p. 5920 - 5923 (2009/04/05)
The synthesis of an oligosaccharide libr...
1651-41-8 Process route
-
-
C31H29F17O11

-
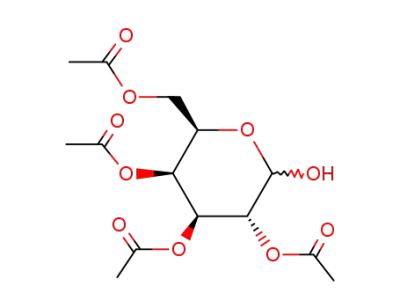
-
3947-62-4,6207-76-7,10343-06-3,19235-21-3,22554-70-7,22860-22-6,57884-82-9,62057-79-8,70191-05-8,109525-54-4,140147-37-1,47339-09-3
2,3,4,6-tetra-O-acetyl-α/β-D-galactopyranose

-
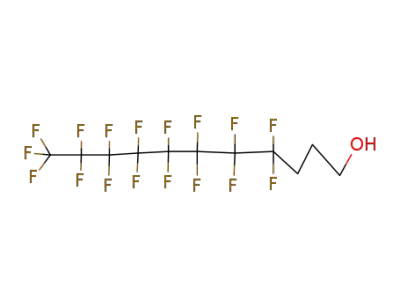
-
1651-41-8
4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,11-heptadecafluoroundecan-1-ol
| Conditions | Yield |
|---|---|
|
With
ammonium cerium (IV) nitrate;
In
MeCN; water;
at 20 ℃;
for 2.5h;
|
78% 64% |
-
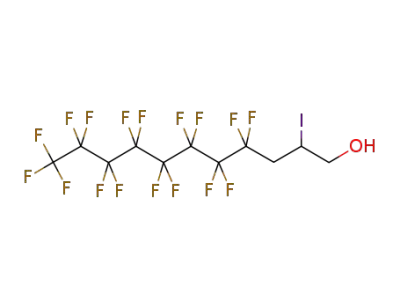
-
38550-45-7
3-perfluoro-n-octyl-2-iodo-1-propanol

-
-
1651-41-8
4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,11-heptadecafluoroundecan-1-ol
| Conditions | Yield |
|---|---|
|
With
tri-n-butyl-tin hydride;
|
88% |
|
With
tri-n-butyl-tin hydride;
In
toluene;
for 8h;
Heating;
|
80% |
|
With
hydrazine hydrate;
nickel;
In
methanol;
at 0 - 10 ℃;
for 2h;
|
78% |
|
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
|
76% |
|
With
tri-n-butyl-tin hydride;
In
various solvent(s);
|
75% |
|
With
2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride;
In
various solvent(s);
at 80 ℃;
for 4h;
|
75% |
|
With
2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride;
at 80 ℃;
for 24h;
|
75% |
|
With
2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride;
at 80 ℃;
for 24h;
|
70% |
|
3-perfluoro-n-octyl-2-iodo-1-propanol;
With
lithium aluminium tetrahydride;
In
diethyl ether;
at 20 ℃;
Inert atmosphere;
With
water; sodium hydroxide;
In
diethyl ether;
|
45% |
|
With
nickel; hydrazine hydrate;
In
methanol;
at 0 - 10 ℃;
for 2h;
|
|
|
With
2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride;
In
toluene;
at 60 - 85 ℃;
for 12.25h;
|
1651-41-8 Upstream products
-
38550-45-7
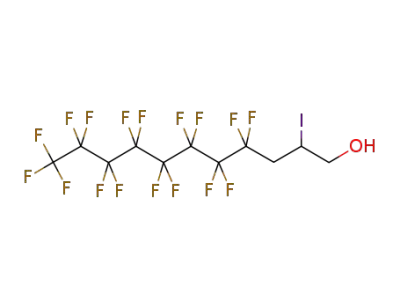
3-perfluoro-n-octyl-2-iodo-1-propanol
-
507-63-1
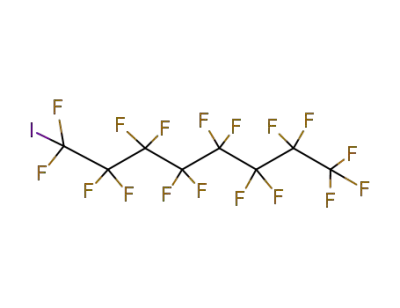
1-iodoheptadecafluorooctane
-
107-18-6
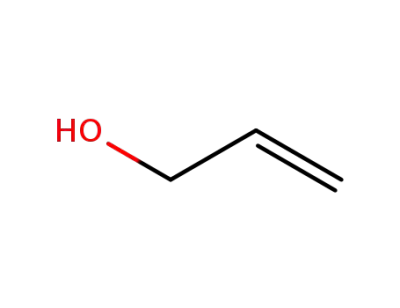
allyl alcohol
1651-41-8 Downstream products
-
200112-75-0
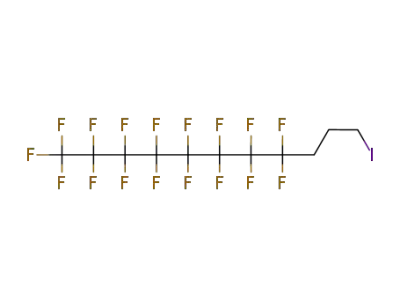
3-(perfluorooctyl)propyl iodide
-
228570-07-8
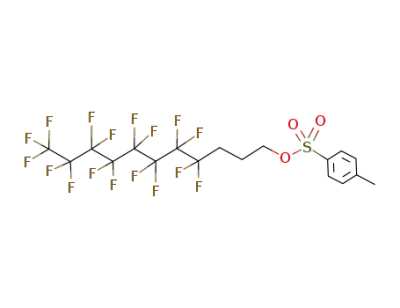
toluene-4-sulfonic acid 4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,11-heptadecafluoro-undecyl ester
-
34598-33-9
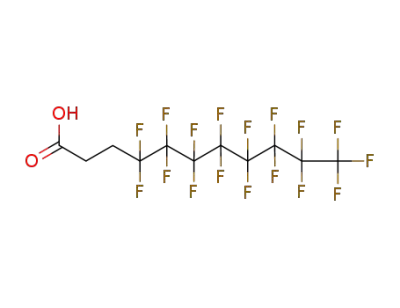
4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,11-heptadecafluoroundecanoic acid
-
512169-29-8
N-benzyloxy-2-(4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,11-heptadecafluoro-undecyloxy)-N-(3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluoro-octyl)-acetamide
Relevant Products
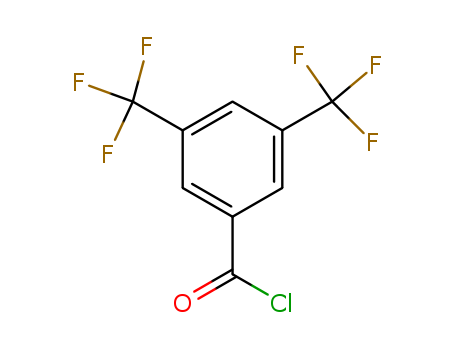
-
3,5-Bis(Trifluoromethyl)Benzoyl Chloride
CAS:785-56-8
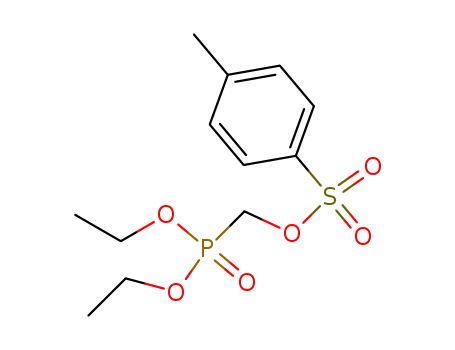
-
Diethyl P-Toluene Sulfonyloxy Methyl Phosphonate(Desmp)
CAS:31618-90-3
-
2,2,3,4,4,4-Hexafluorobutyl Methacrylate
CAS:36405-47-7