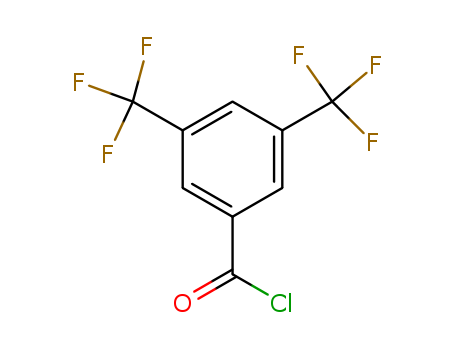
785-56-8
- Product Name:3,5-Bis(Trifluoromethyl)Benzoyl Chloride
- MF:C9H3ClF6O
- Purity:99%
- Molecular Weight:276.566
Product Details
Bulk supply high purity 3,5-Bis(Trifluoromethyl)Benzoyl Chloride 785-56-8, Paid sample available
- Molecular Formula:C9H3ClF6O
- Molecular Weight:276.566
- Appearance/Colour:clear colorless to very slightly yellow liquid
- Vapor Pressure:0.0865mmHg at 25°C
- Melting Point:41760oC
- Refractive Index:n20/D 1.435(lit.)
- Boiling Point:194.3 °C at 760 mmHg
- Flash Point:72.2 °C
- PSA:17.07000
- Density:1.526 g/cm3
- LogP:4.10320
3,5-Bis(trifluoromethyl)benzoyl chloride(Cas 785-56-8) Usage
InChI:InChI=1/C10H4Cl3F17Si/c11-31(12,13)2-1-3(14,15)4(16,17)5(18,19)6(20,21)7(22,23)8(24,25)9(26,27)10(28,29)30/h1-2H2
785-56-8 Relevant articles
Cyano-and ketone-containing selenoesters as multi-target compounds against resistant cancers
Alonso-Martínez, Francisco-Javier,Benito-Lama, Miguel,Dobiasová, Simona,Domínguez-álvarez, Enrique,Habibullah, Giyaullah,Kincses, Annamária,Nové, Márta,Salardón-Jiménez, Noemi,Sevilla-Hernández, Clotilde,Spengler, Gabriella,Szemerédi, Nikoletta,Viktorová, Jitka
, (2021/09/13)
Fifteen selenocompounds, comprising of e...
Nickel-Mediated Photoreductive Cross Coupling of Carboxylic Acid Derivatives for Ketone Synthesis**
Brauer, Jan,Quraishi, Elisabeth,Kammer, Lisa Marie,Opatz, Till
supporting information, p. 18168 - 18174 (2021/11/30)
A simple visible light photochemical, ni...
Isoalantolactone derivative, pharmaceutical composition and application thereof
-
Paragraph 0014, (2019/02/02)
The invention relates to an isoalantolac...
Chemo- and Regioselective Functionalization of Isotactic Polypropylene: A Mechanistic and Structure-Property Study
Williamson, Jill B.,Na, Christina G.,Johnson, Robert R.,Daniel, William F. M.,Alexanian, Erik J.,Leibfarth, Frank A.
supporting information, p. 12815 - 12823 (2019/08/20)
Polyolefins represent a high-volume clas...
785-56-8 Process route
-
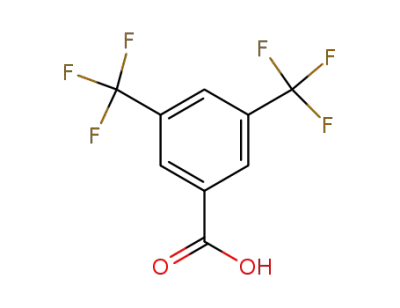
-
725-89-3
3,5-bistrifluoromethylbenzoic acid

-
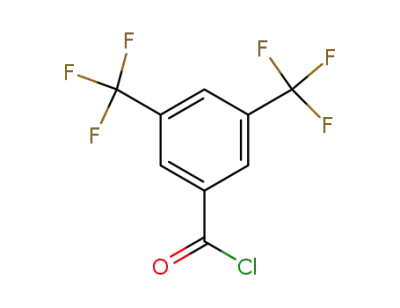
-
785-56-8
3,5-bis(trifluoromethyl)phenyl carboxylic acid chloride
| Conditions | Yield |
|---|---|
|
With
oxalyl dichloride;
In
dichloromethane; N,N-dimethyl-formamide;
at 0 ℃;
for 1h;
|
100% |
|
With
thionyl chloride;
for 5h;
Reflux;
|
54% |
|
With
thionyl chloride;
|
|
|
With
oxalyl dichloride; N,N-dimethyl-formamide;
In
tetrahydrofuran;
Ambient temperature;
|
|
|
With
oxalyl dichloride; N,N-dimethyl-formamide;
In
dichloromethane;
|
|
|
With
thionyl chloride;
|
|
|
With
thionyl chloride;
at 80 ℃;
for 4h;
|
|
|
With
thionyl chloride;
In
chloroform;
at 20 ℃;
for 1.25h;
Heating / reflux;
|
|
|
With
thionyl chloride;
N,N-dimethyl-formamide;
In
chloroform;
at 20 ℃;
for 1.25h;
Product distribution / selectivity;
Heating / reflux;
|
|
|
With
oxalyl dichloride;
N,N-dimethyl-formamide;
In
dichloromethane;
at 0 - 20 ℃;
for 4.5h;
|
|
|
With
thionyl chloride;
In
chloroform;
at 20 ℃;
for 1.25h;
Product distribution / selectivity;
Reflux;
|
|
|
With
thionyl chloride;
for 4h;
Reflux;
|
|
|
With
oxalyl dichloride; N,N-dimethyl-formamide;
In
dichloromethane;
at 0 - 20 ℃;
for 1.6h;
Inert atmosphere;
|
|
|
With
oxalyl dichloride; N,N-dimethyl-formamide;
In
dichloromethane;
at 0 - 20 ℃;
for 4h;
|
|
|
With
thionyl chloride;
|
|
|
With
oxalyl dichloride; N,N-dimethyl-formamide;
In
dichloromethane;
at 0 - 20 ℃;
for 1.75h;
Inert atmosphere;
|
|
|
With
thionyl chloride;
for 2h;
Reflux;
|
|
|
With
oxalyl dichloride; N,N-dimethyl-formamide;
In
dichloromethane;
at 0 - 20 ℃;
|
|
|
With
thionyl chloride;
at 20 - 85 ℃;
for 3h;
Inert atmosphere;
|
|
|
With
thionyl chloride;
In
1,2-dichloro-ethane;
at 90 ℃;
for 4h;
Temperature;
|
|
|
With
oxalyl dichloride;
In
dichloromethane; N,N-dimethyl-formamide;
for 2h;
|
|
|
With
oxalyl dichloride; N,N-dimethyl-formamide;
In
dichloromethane;
at 0 ℃;
|
|
|
With
oxalyl dichloride; N,N-dimethyl-formamide;
In
dichloromethane;
|
|
|
With
thionyl chloride; N,N-dimethyl-formamide;
In
ethyl acetate;
for 3h;
Inert atmosphere;
Reflux;
|
-
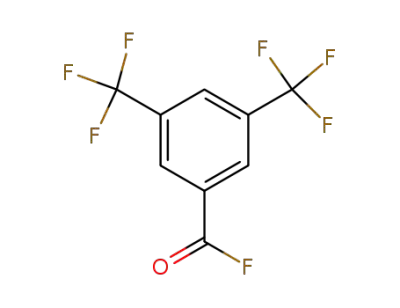
-
401-96-7
3,5-bis(trifluoromethyl)benzoyl fluoride

-

-
785-56-8
3,5-bis(trifluoromethyl)phenyl carboxylic acid chloride
| Conditions | Yield |
|---|---|
|
With
tetrachlorosilane; triphenylphosphine;
aluminium trichloride;
|
|
|
With
tetrachlorosilane;
aluminum (III) chloride;
at 40 ℃;
for 4h;
|
785-56-8 Upstream products
-
725-89-3

3,5-bistrifluoromethylbenzoic acid
-
402-31-3
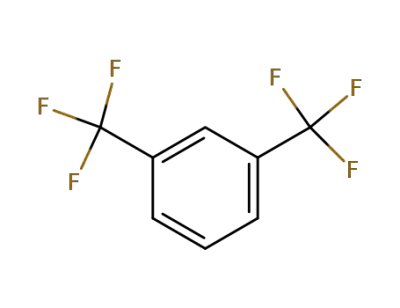
1,3-bis(trifluoromethyl)benzene
-
328-70-1
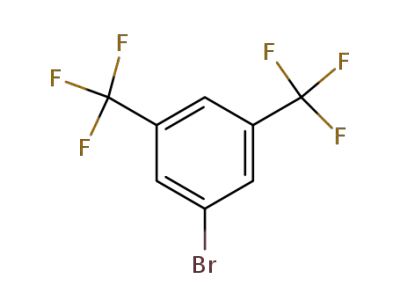
3,6-bis(trifluoromethyl)bromobenzene
-
401-96-7

3,5-bis(trifluoromethyl)benzoyl fluoride
785-56-8 Downstream products
-
30071-94-4
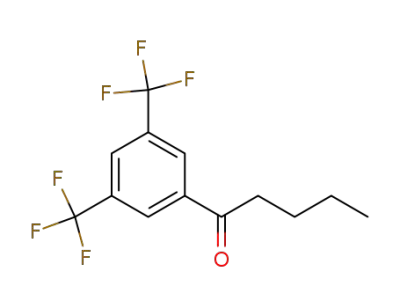
3,5-bis(trifluoromethyl)phenyl n-butyl ketone
-
30071-93-3
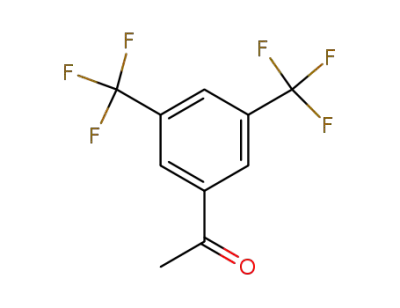
3,5-bis(trifluoromethyl)phenyl methyl ketone
-
863-99-0
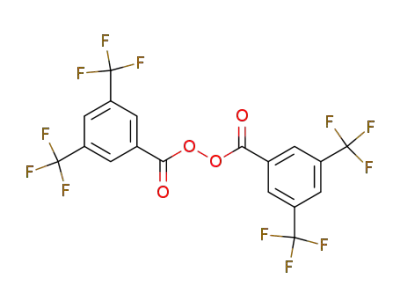
Di-<3.5-bis-trifluormethyl-benzoyl>-peroxyd
-
20929-20-8
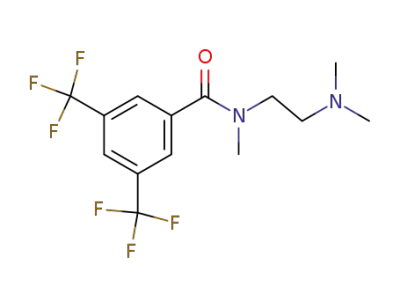
3,5-Di-(trifluormethyl)-benzoesaeure-N-methyl-N-<2-dimethylamino-ethyl>-amid
Relevant Products
-
3,5-Bis(Trifluoromethyl)Benzyl Chloride
CAS:75462-59-8
-
1,6-Divinylperfluorohexane
CAS:1800-91-5
-
Isphenol A-Type Diethyl Ether Dianhydride
CAS:38103-06-9