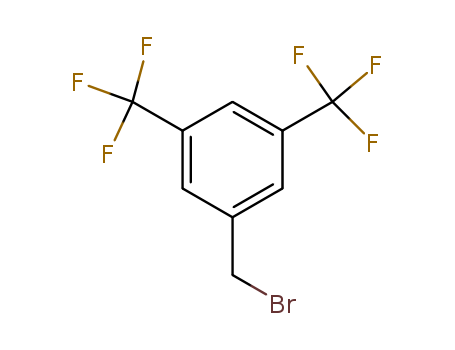
32247-96-4
- Product Name:3,5-Bis(Trifluoromethyl)Benzyl Bromide
- MF:C9H5BrF6
- Purity:99%
- Molecular Weight:307.033
Product Details
Good factory exports good 3,5-Bis(Trifluoromethyl)Benzyl Bromide 32247-96-4
- Molecular Formula:C9H5BrF6
- Molecular Weight:307.033
- Appearance/Colour:colorless to yellow brown Clear liquid
- Vapor Pressure:1.34mmHg at 25°C
- Melting Point:18°C
- Refractive Index:n20/D 1.445(lit.)
- Boiling Point:178.3 °C at 760 mmHg
- Flash Point:26.1 °C
- PSA:0.00000
- Density:1.651 g/cm3
- LogP:4.61910
3,5-Bis(trifluoromethyl)benzyl bromide(Cas 32247-96-4) Usage
|
Synthesis |
Synthesis of 3, 5-bis (trifluoromethyl)-benzyl bromide:In a 4-neck flask with capacity 1000 ml equipped with mechanical agitator, thermometer, bubble condenser and 100 ml loading funnel, 262.2 g of the product of Example 1 (ii) at 92. 8% (0. 988 moles), 550,2 g HBr 48% (3.2645 moles) are loaded; this is heated at 50°C so as to melt the alcohol, then one starts to dose 113 g of concentrated His04 (1.153 moles). Pouring is accomplished in 30 minutes, noting an increase of the internal temperature. This is heated to 100-105°C and left to react for 8 hours. The reaction is completed by reflux heating for per 1.5 hours. the mixture is left to settle and the phases are separated; the solvents are removed from the organic phase and the product in the title is obtained with a yield of 99.1 %. |
InChI:InChI=1/C9H5BrF6/c10-4-5-1-6(8(11,12)13)3-7(2-5)9(14,15)16/h1-3H,4H2
32247-96-4 Relevant articles
One-Step Synthesis of Substituted Benzofurans from ortho- Alkenylphenols via Palladium-Catalyzed C=H Functionalization
Yang, Dejun,Zhu, Yifei,Yang, Na,Jiang, Qiangqiang,Liu, Renhua
supporting information, p. 1731 - 1735 (2016/06/09)
A dehydrogenative oxygenation of C(sp2)=...
PROCESS FOR THE PREPARATION 3,5-BIS(TRIFLUOROMETHYL)BENZYLALCOHOL
-
Page/Page column 7-8, (2010/02/11)
The present invention concerns a process...
N-acylamino benzyl ether derivatives
-
, (2008/06/13)
This invention relates to N-acylamino ar...
Scavenger assisted combinatorial process for preparing libraries of tertiary amine compounds
-
, (2008/06/13)
This invention relates to a novel soluti...
32247-96-4 Process route
-
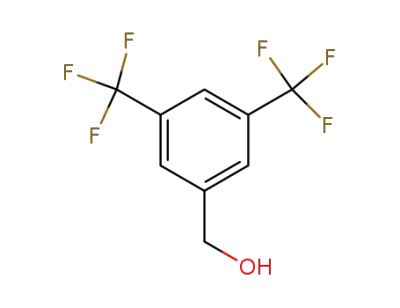
-
32707-89-4
3,5-bis(trifluoromethyl)benzenemethanol

-
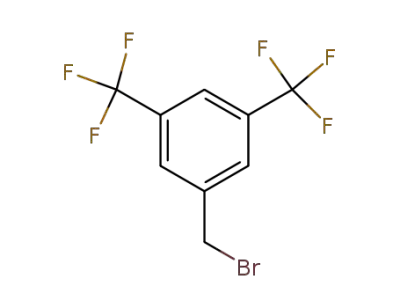
-
32247-96-4
3,5-bis(trifluoromethyl)benzyl bromide
| Conditions | Yield |
|---|---|
|
With
sulfuric acid; hydrogen bromide;
In
water;
at 50 - 105 ℃;
for 10h;
Heating / reflux;
|
99.1% |
|
With
phosphorus tribromide;
In
dichloromethane;
for 0.5h;
Cooling with ice;
|

-

-
32247-96-4
3,5-bis(trifluoromethyl)benzyl bromide
| Conditions | Yield |
|---|---|
|
|
|
|
|
|
|
|
32247-96-4 Upstream products
-
32707-89-4

3,5-bis(trifluoromethyl)benzenemethanol
32247-96-4 Downstream products
-
132130-91-7
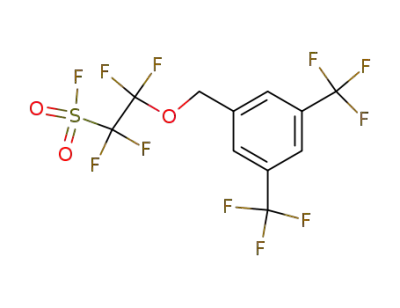
2-(3,5-Bis-trifluoromethyl-benzyloxy)-1,1,2,2-tetrafluoro-ethanesulfonyl fluoride
-
754-41-6
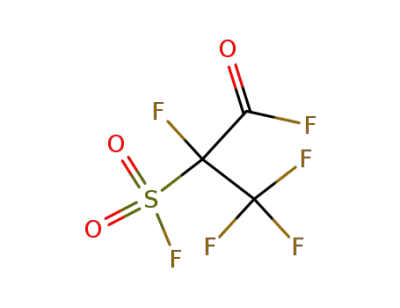
2-(fluorosulfonyl)tetrafluoropropionyl fluoride
-
132130-92-8
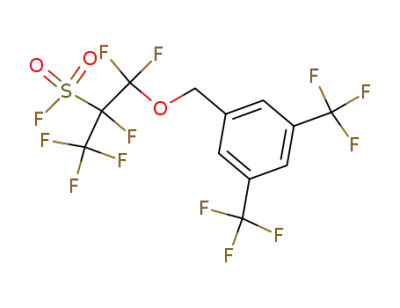
1-(3,5-Bis-trifluoromethyl-benzyloxy)-1,1,2,3,3,3-hexafluoro-propane-2-sulfonyl fluoride
-
169673-06-7
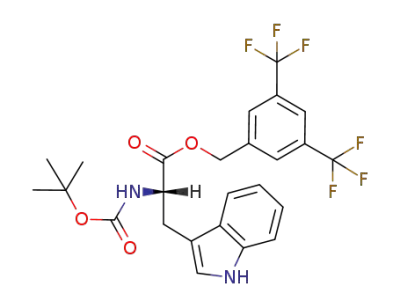
3,5-bis(trifluoromethyl)benzyl N-(tert-butoxycarbonyl)-L-tryptophanate
Relevant Products
-
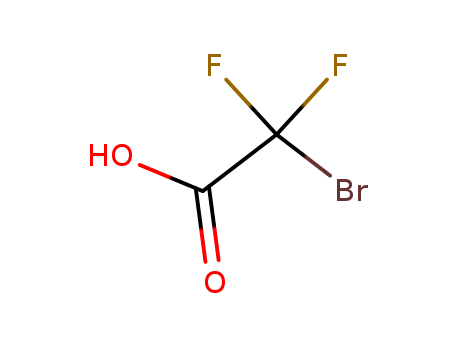
Bromodifluoroacetic Acid
CAS:354-08-5
-
Methyl Perfluoro(5-Methyl-4,7- Dioxanon-8-Enoate
CAS:63863-43-4