63863-43-4
- Product Name:Methyl Perfluoro(5-Methyl-4,7- Dioxanon-8-Enoate
- MF:C9H3 F13 O4
- Purity:99%
- Molecular Weight:422.1
Product Details
Good factory exports good Methyl Perfluoro(5-Methyl-4,7- Dioxanon-8-Enoate 63863-43-4
- Molecular Formula:C9H3 F13 O4
- Molecular Weight:422.1
- Vapor Pressure:0.00961mmHg at 25°C
- Refractive Index:1.317
- Boiling Point:151
- Flash Point:110.4°C
- PSA:44.76000
- Density:1.592
- LogP:4.27670
METHYL PERFLUORO(5-METHYL-4,7-DIOXANON-8-ENOATE)(Cas 63863-43-4) Usage
|
General Description |
Methyl Perfluoro(5-methyl-4,7-dioxanon-8-enoate) is a chemical compound recognized for its long name and complex structure. As a perfluorinated compound, it is referenced in the category of chemicals that includes carbon-fluorine bonds, known for their strong and stable nature. Perfluorinated compounds are extensively used in industrial applications due to their resistance to heat, oil, and water. This specific compound, the methyl perfluoro(5-methyl-4,7-dioxanon-8-enoate), has potential applications in the production of various consumer goods and industrial products due to its properties. Due to the complex nature and potential implications on human health and the environment, it's use and disposal are likely regulated in many regions. |
InChI:InChI=1/C9H3F13O4/c1-24-4(23)5(13,14)8(19,20)26-6(15,7(16,17)18)9(21,22)25-3(12)2(10)11/h1H3
63863-43-4 Relevant articles
Process for producing fluorinated vinyl ether
-
Page 6, (2008/06/13)
There is provided a process for producin...
Alkyl perfluoro-ω-fluoroformyl esters and monomers therefrom
-
, (2008/06/13)
Alkyl perfluoro-ω-fluoroformyl esters ar...
Alkyl perfluoro-ω-fluoroformyl esters and their preparation
-
, (2008/06/13)
Alkyl perfluoro-ω-fluoroformyl esters ar...
63863-43-4 Process route
-
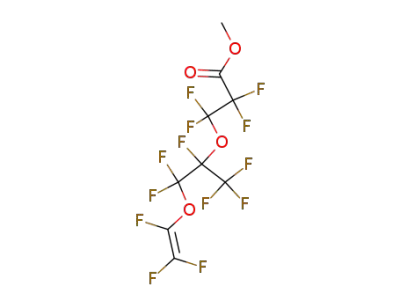
![2,2,3,3-Tetrafluoro-3-[1,2,2-trifluoro-2-(1,2,2,2-tetrafluoro-1-fluorocarbonyl-ethoxy)-1-trifluoromethyl-ethoxy]-propionic acid methyl ester](/upload/2025/12/6fc4f30e-3d43-427b-ab34-9c1f2bed11fd.png)
-
69116-73-0
2,2,3,3-Tetrafluoro-3-[1,2,2-trifluoro-2-(1,2,2,2-tetrafluoro-1-fluorocarbonyl-ethoxy)-1-trifluoromethyl-ethoxy]-propionic acid methyl ester

-
-
155435-62-4
C11H6F14O6

-
-
63863-43-4
methyl 6-trifluoroethenoxy-5-trifluoromethyl-4-oxa-2,2,3,3,5,6,6-heptafluorohexanoate
| Conditions | Yield |
|---|---|
|
With
potassium carbonate;
at 220 ℃;
|
61% |
-
-
C10H3F14O6(1-)*K(1+)

-
-
155435-62-4
C11H6F14O6

-

-
63863-43-4
methyl 6-trifluoroethenoxy-5-trifluoromethyl-4-oxa-2,2,3,3,5,6,6-heptafluorohexanoate
| Conditions | Yield |
|---|---|
|
at 160 - 200 ℃;
for 6h;
under 19.502 - 97.5098 Torr;
Pyrolysis;
|
89 - 95 %Chromat. 1 - 4 %Chromat. |
63863-43-4 Upstream products
-
69116-73-0
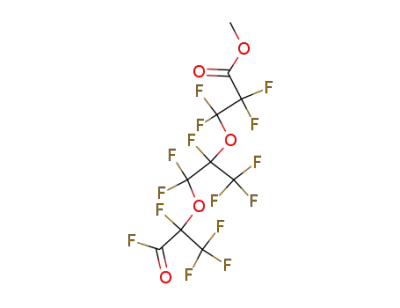
2,2,3,3-Tetrafluoro-3-[1,2,2-trifluoro-2-(1,2,2,2-tetrafluoro-1-fluorocarbonyl-ethoxy)-1-trifluoromethyl-ethoxy]-propionic acid methyl ester
63863-43-4 Downstream products
-
144373-69-3
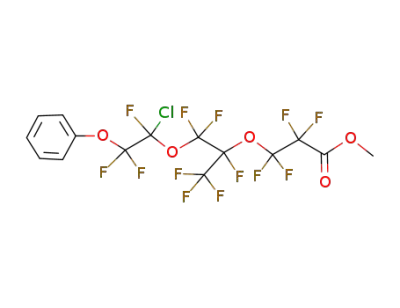
3-[2-(1-Chloro-1,2,2-trifluoro-2-phenoxy-ethoxy)-1,2,2-trifluoro-1-trifluoromethyl-ethoxy]-2,2,3,3-tetrafluoro-propionic acid methyl ester
-
133573-37-2
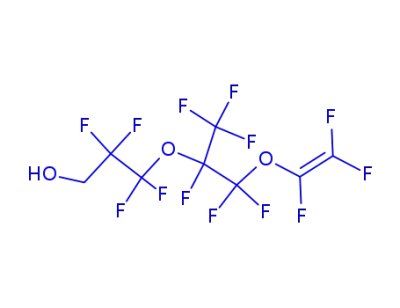
9,9-dihydro-9-hydroxyperfluoro-3,6-dioxa-5-methyl-1-nonene
-
144373-64-8
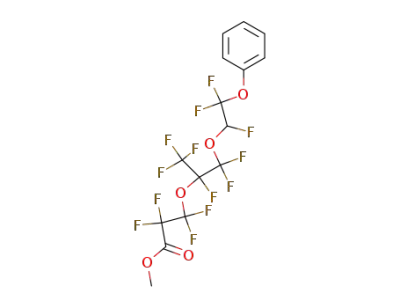
2,2,3,3-Tetrafluoro-3-[1,2,2-trifluoro-1-trifluoromethyl-2-(1,2,2-trifluoro-2-phenoxy-ethoxy)-ethoxy]-propionic acid methyl ester
-
69804-18-8
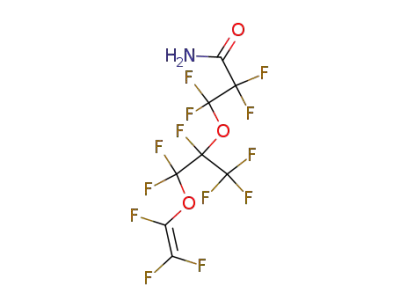
2,2,3,3-Tetrafluoro-3-(1,2,2-trifluoro-1-trifluoromethyl-2-trifluorovinyloxy-ethoxy)-propionamide
Relevant Products
-
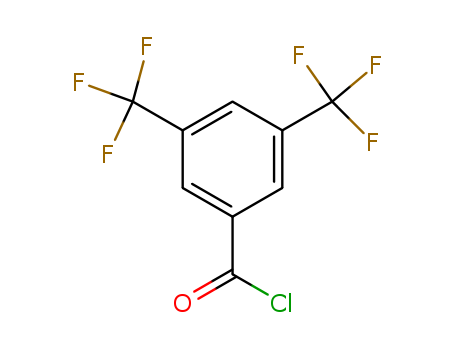
3,5-Bis(Trifluoromethyl)Benzoyl Chloride
CAS:785-56-8
-
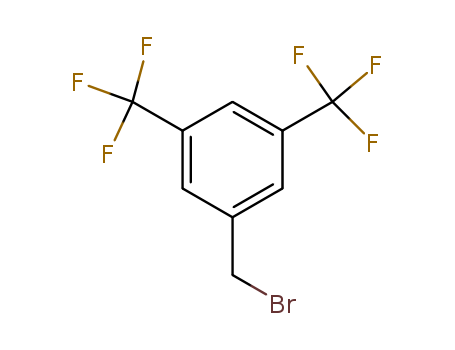
3,5-Bis(Trifluoromethyl)Benzyl Bromide
CAS:32247-96-4