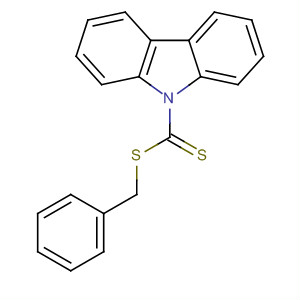
27249-90-7
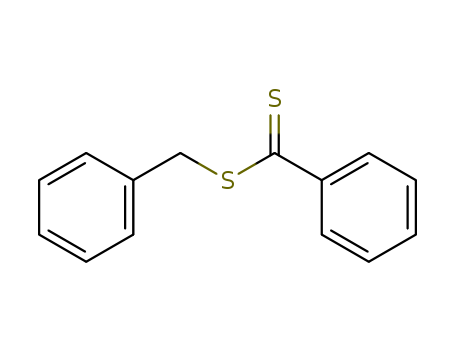
- Product Name:Benzyl Benzodithioate
- MF:C14H12 S2
- Purity:99%
- Molecular Weight:244.381
Product Details
High quality purity >99% Benzyl Benzodithioate 27249-90-7 for sale
- Molecular Formula:C14H12 S2
- Molecular Weight:244.381
- Vapor Pressure:0mmHg at 25°C
- Melting Point:55℃
- Refractive Index:1.67
- Boiling Point:372.9°Cat760mmHg
- Flash Point:179.3°C
- PSA:57.39000
- Density:1.2g/cm3
- LogP:4.29550
BENZYL BENZODITHIOATE(Cas 27249-90-7) Usage
|
General Description |
Need help choosing the correct RAFT Agent? Please consult the RAFT Agent to Monomer compatibility table. |
InChI:InChI=1/C14H12S2/c15-14(13-9-5-2-6-10-13)16-11-12-7-3-1-4-8-12/h1-10H,11H2
27249-90-7 Relevant articles
Nanostructured thermosensitive polymers with radical scavenging ability
Zhou, Guangchang,Harruna, Issifu I.,Zhou, Weilie L.,Aicher, Wilhelm K.,Geckeler, Kurt E.
, p. 569 - 573 (2007)
The thermosensitive [60]fullerene end-ca...
A facile one pot strategy for the synthesis of well-defined polyacrylates from acrylic acid via RAFT polymerization
Li, Qianbiao,Wang, Taisheng,Dai, Jingwen,Ma, Chao,Jin, Bangkun,Bai, Ruke
, p. 3331 - 3334 (2014)
A facile one pot strategy for the prepar...
Thermosensitive Gold Nanoparticles
Zhu, Ming-Qiang,Wang, Li-Qiong,Exarhos, Gregory J.,Li, Alexander D. Q.
, p. 2656 - 2657 (2004)
Thermosensitive gold nanoparticles were ...
Styrene-vinyl pyridine diblock copolymers: Synthesis by RAFT polymerization and self-assembly in solution and in the bulk
Zamfir, Mirela,Patrickios, Costas S.,Montagne, Franck,Abetz, Clarissa,Abetz, Volker,Oss-Ronen, Liat,Talmon, Yeshayahu
, p. 1636 - 1644 (2012)
Reversible addition-fragmentation chain ...
Compound embodiments that release H2S by reaction with a reactive compound and methods of making and using the same
-
Page/Page column 39; 41-43, (2021/08/04)
Disclosed herein are embodiments of a do...
Regioselective reaction of fluorinated aryllithium reagents and carbon disulfide in cyclopentyl methyl ether: Efficient synthesis of dithioesters and liquid crystal compounds having a difluoromethyleneoxy moiety
Araki, Keisuke,Fuchigami, Tsugumichi,Gotoh, Yasuyuki,Inoue, Munenori,Maebayashi, Haruki
, (2020/03/25)
Regioselective reaction (carbophilic ove...
Ynamide-Mediated Thionoester and Dithioester Syntheses
Yao, Chaochao,Yang, Jinhua,Lu, Xiaobiao,Zhang, Shuyu,Zhao, Junfeng
, p. 6628 - 6631 (2020/09/02)
A novel ynamide-mediated synthesis of th...
Copper-catalyzed cross-coupling of thiols, alcohols, and oxygen for the synthesis of esters
Lim, Seungyeon,Ji, Miran,Wang, Xi,Lee, Chan,Jang, Hye-Young
supporting information, p. 591 - 595 (2015/01/30)
Copper-catalyzed, one-pot, three-compone...
27249-90-7 Process route
-
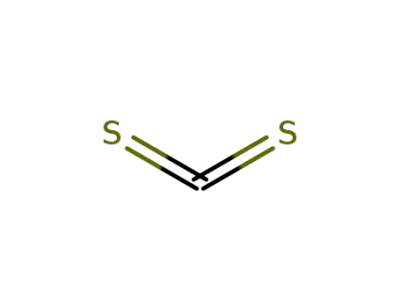
-
75-15-0,12122-00-8
carbon disulfide

-
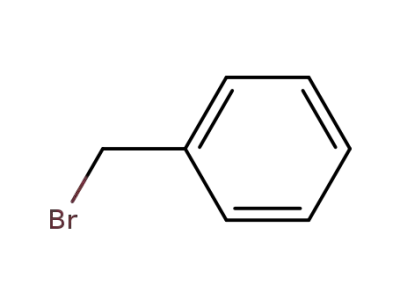
-
100-39-0
benzyl bromide

-
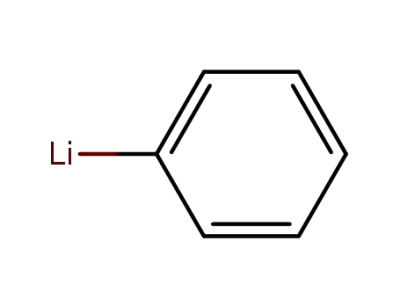
-
591-51-5
phenyllithium

-
-
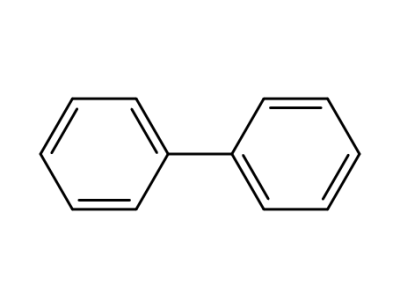
92-52-4,1594-86-1
biphenyl

-
-
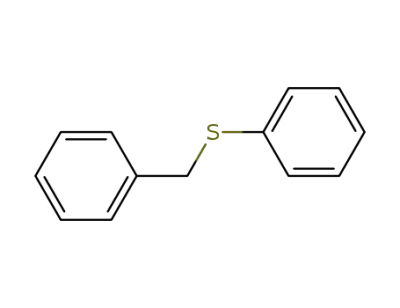
831-91-4
Benzyl phenyl sulfide

-
-
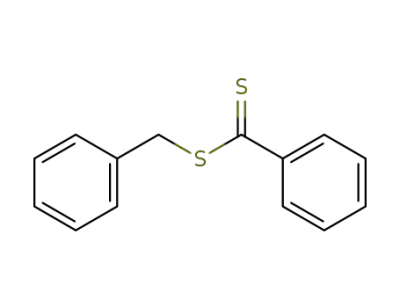
27249-90-7
benzyl dithiobenzoate

-
-
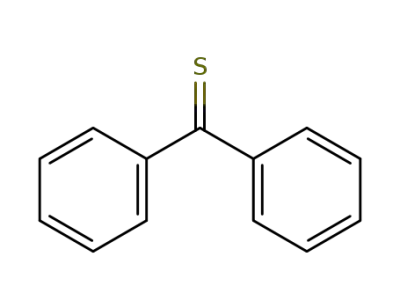
1450-31-3
Thiobenzophenon
| Conditions | Yield |
|---|---|
|
carbon disulfide; phenyllithium;
With
copper(l) cyanide;
In
dibutyl ether;
at -50 - 20 ℃;
for 1h;
Schlenk technique;
Inert atmosphere;
benzyl bromide;
In
dibutyl ether; N,N-dimethyl-formamide;
at 20 ℃;
for 4h;
Solvent;
|
79.1 %Chromat. 1.4 %Chromat. |
|
carbon disulfide; phenyllithium;
With
copper(l) cyanide;
In
diethylene glycol dimethyl ether;
at -50 - 20 ℃;
for 1h;
Schlenk technique;
Inert atmosphere;
benzyl bromide;
In
dibutyl ether; diethylene glycol dimethyl ether;
at 20 ℃;
for 4h;
|
31.5 %Chromat. 50.2 %Chromat. |
-
-
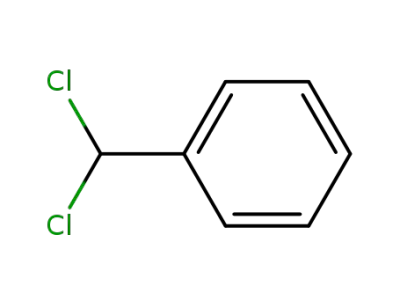
98-87-3
benzylidene dichloride

-
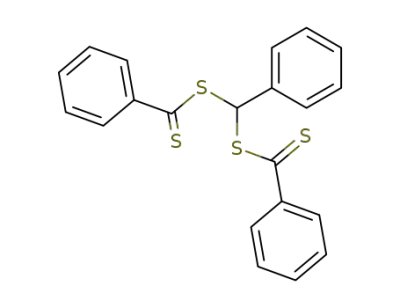
-
benzylidene bis-dithiobenzoate

-
-
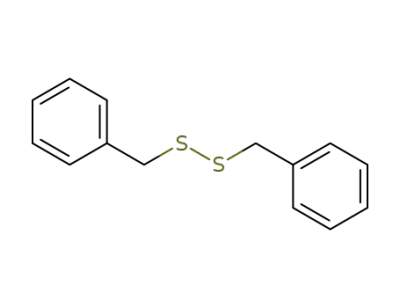
150-60-7
dibenzyl disulphide

-

-
27249-90-7
benzyl dithiobenzoate
| Conditions | Yield |
|---|---|
|
With
sodium sulfide;
In
ethanol;
Heating;
|
39% 36% 6% |
27249-90-7 Upstream products
-
121-68-6
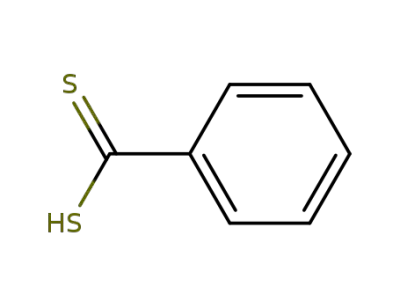
dithiobenzoic acid
-
100-44-7
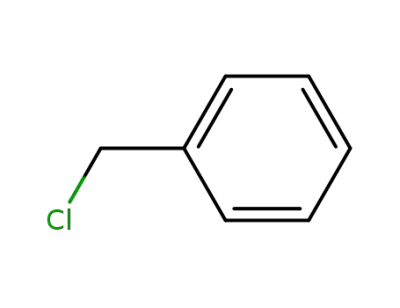
benzyl chloride
-
13402-51-2
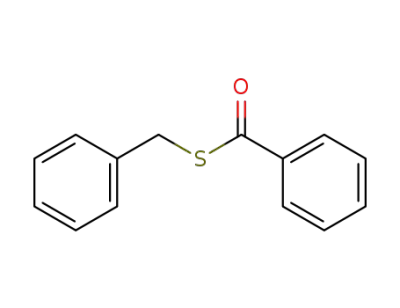
benzyl benzothioate
-
100-53-8
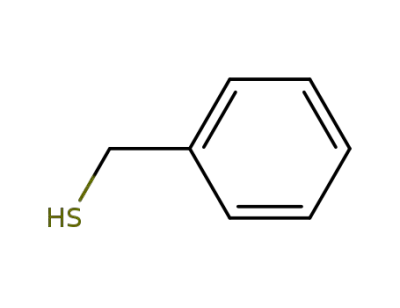
phenylmethanethiol
27249-90-7 Downstream products
-
137715-21-0
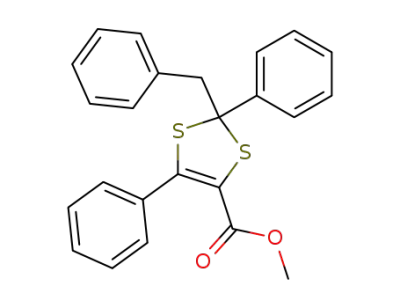
2-benzyl-2,5-diphenyl-4-methoxycarbonyl-1,3-dithiole
-
766-92-7
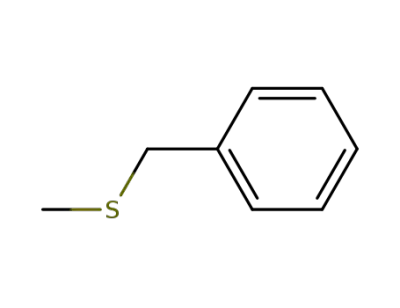
[(methylthio)methyl]-benzene
-
2168-78-7
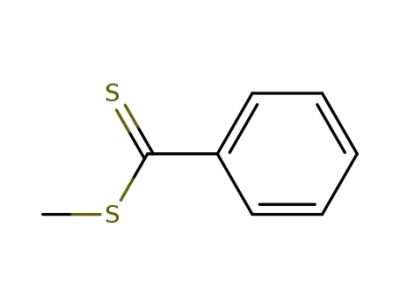
methyl dithiobenzoate
-
501-65-5
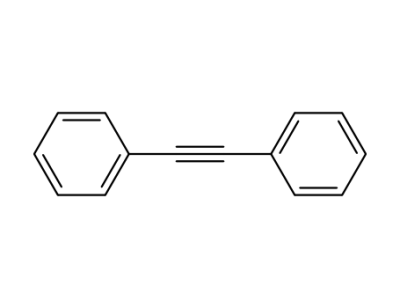
diphenyl acetylene
Relevant Products
-
1-Fluoropyridinium Triflate
CAS:107263-95-6
-
Zyl 9H-Carbazole-9-Carbodithioate
CAS:137780-73-5
-
2-Methyl-2-Propylbenzodithiolate
CAS:5925-55-3