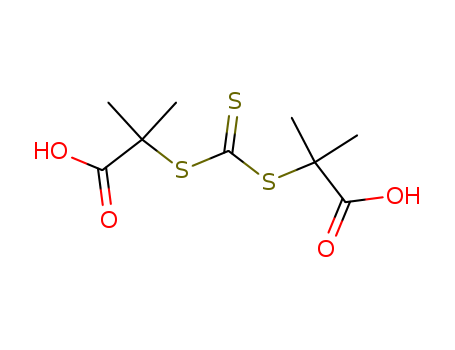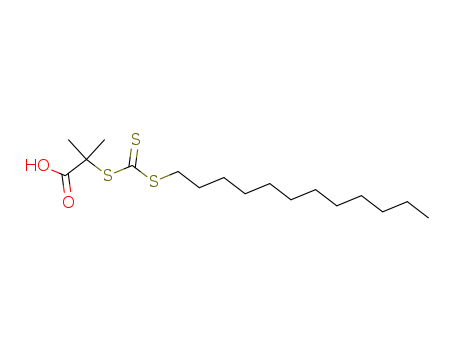
461642-78-4
- Product Name:2-(Dodecylsulfanylthiocarbonylsulfanyl)-2-Methylpropionic Acid
- MF:C17H32O2S3
- Purity:99%
- Molecular Weight:364.638
Product Details
Buy high quality and low price 2-(Dodecylsulfanylthiocarbonylsulfanyl)-2-Methylpropionic Acid 461642-78-4 now
- Molecular Formula:C17H32O2S3
- Molecular Weight:364.638
- Appearance/Colour:pale yellow solid
- Vapor Pressure:0mmHg at 25°C
- Melting Point:62-64℃ (hexane )
- Boiling Point:505.984°C at 760 mmHg
- PKA:2.89±0.10(Predicted)
- Flash Point:259.81°C
- PSA:119.99000
- Density:1.083g/cm3
- LogP:6.52180
2-(DODECYLSULFANYLTHIOCARBONYLSULFANYL)-2-METHYLPROPIONIC ACID(Cas 461642-78-4) Usage
|
General Description |
Need help choosing the correct RAFT Agent? Please consult the RAFT Agent to Monomer compatibility table. |
|
Industrial uses |
The trithiocarbonates have a structure that contains three sulfur atoms. All three sulfur atoms can participate in bonding to the mineral surfaces. Philips Petroleum Company did most of the development of this class of collectors. Monoalkyl trithiocarbonate (Orfom 800 series) is used as a collector in flotation of copper and copper–lead ores. |
InChI:InChI=1/C17H32O2S3/c1-4-5-6-7-8-9-10-11-12-13-14-21-16(20)22-17(2,3)15(18)19/h4-14H2,1-3H3,(H,18,19)
461642-78-4 Relevant articles
Initiator-chain transfer agent combo in the RAFT polymerization of styrene
Wu, Ying,Zhang, Wei,Zhang, Zhengbiao,Pan, Xiangqiang,Cheng, Zhenping,Zhu, Jian,Zhu, Xiulin
, p. 9722 - 9724 (2014)
The combo agent with roles of initiator ...
Preparation of thermoresponsive polymers bearing amino acid diamide derivatives via RAFT polymerization
Liu, Zhilei,Hu, Jiwen,Sun, Jianping,He, Guping,Li, Yinghui,Zhang, Ganwei
, p. 3573 - 3586 (2010)
We report here the synthesis of well-def...
Nanocrystalline cellulose grafted random copolymers of N- isopropylacrylamide and acrylic acid synthesized by RAFT polymerization: Effect of different acrylic acid contents on LCST behavior
Zeinali, Elnaz,Haddadi-Asl, Vahid,Roghani-Mamaqani, Hossein
, p. 31428 - 31442 (2014)
Free and nanocrystalline cellulose (NCC)...
Dual electrical switching permeability of vesicles via redox-responsive self-assembly of amphiphilic block copolymers and polyoxometalates
Chong, Dandan,Tan, Junyan,Zhang, Jinlong,Zhou, Yue,Wan, Xinhua,Zhang, Jie
, p. 7838 - 7841 (2018)
Electro-responsive vesicles were demonst...
Synthesis, characterization, and antibacterial properties of a hydroxyapatite adhesive block copolymer
Zhang, Qiang Matthew,Serpe, Michael J.
, p. 8018 - 8025 (2014)
A novel diblock copolymer composed of bi...
Homopolymer vesicles with a gradient bilayer membrane as drug carriers
Fan, Lang,Lu, Hang,Zou, Kaidian,Chen, Jing,Du, Jianzhong
, p. 11521 - 11523 (2013)
We report an unusual homopolymer vesicle...
Preparation of hyperbranched polystyrene-g-poly(N-isopropylacrylamide) copolymers and its application to novel thermo-responsive cell culture dishes
Sudo, Yu,Sakai, Hideaki,Nabae, Yuta,Hayakawa, Teruaki,Kakimoto, Masa-Aki
, p. 307 - 314 (2015)
This paper describes the preparation of ...
One-step synthesis of pegylated cationic nanogels of poly(N,N′-dimethylaminoethyl methacrylate) in aqueous solution via self-stabilizing micelles using an amphiphilic macroRAFT agent
Yan, Lifeng,Tao, Wei
, p. 2161 - 2167 (2010)
Cationic nanogels of Pegylated poly(N,N′...
Oxygen-Initiated and Regulated Controlled Radical Polymerization under Ambient Conditions
Lv, Chunna,He, Congze,Pan, Xiangcheng
, p. 9430 - 9433 (2018)
A rapid oxygen-initiated and -regulated ...
Water-soluble and clickable segmented hyperbranched polymers for multifunctionalization and novel architecture construction
Han, Jin,Li, Sipei,Tang, Aijin,Gao, Chao
, p. 4966 - 4977 (2012)
A series of novel and narrowly polydispe...
Isothermal LCST transitions triggered by bioreduction of single polymer end-groups
Summers, Matthew J.,Phillips, Daniel J.,Gibson, Matthew I.
, p. 4223 - 4225 (2013)
The α-termini of RAFT-derived thermoresp...
Folic acid-tethered poly(N-isopropylacrylamide)-phospholipid hybrid nanocarriers for targeted drug delivery
John, Johnson V.,Jeong, Young-Il,Johnson, Renjith P.,Chung, Chung-Wook,Park, Huiju,Kang, Dae Hwan,Cho, Jin Ku,Kim, Yongjin,Kim, Il
, p. 8268 - 8278 (2015)
A series of temperature-responsive lipop...
Hypoxia and temperature dual-stimuli-responsive random copolymers: Facile synthesis, self-assembly and controlled release of drug
Deng, Yinlu,Ji, Chenming,Wu, Yongzhen,Yuan, Hua,Yuan, Weizhong
, p. 10229 - 10238 (2020)
Amphiphilic random copolymers poly(isopr...
2-{[(Dodecylsulfanyl)carbonothioyl]sulfanyl}-2-methyl-propanoic acid: A chain of edge-fused R22(8) and R4 4(20) rings built from O - H...O and C - H...O hydrogen bonds
Zuluaga, Fabio,Grande, Carlos,Cobo, Justo,Glidewell, Christopher
, p. o627-o630 (2010)
In the title compound, C17H32O2S 3, the ...
Mitochondria-targeted polymer-celastrol conjugate with enhanced anticancer efficacy
Geng, Yu,Xiang, Jiajia,Shao, Shiqun,Tang, Jianbin,Shen, Youqing
, p. 122 - 133 (2022/01/11)
Celastrol, a natural triterpene extracte...
Ubiquitous Nature of Rate Retardation in Reversible Addition-Fragmentation Chain Transfer Polymerization
Bradford, Kate G. E.,Petit, Leilah M.,Whitfield, Richard,Anastasaki, Athina,Barner-Kowollik, Christopher,Konkolewicz, Dominik
supporting information, p. 17769 - 17777 (2021/11/10)
Reversible addition-fragmentation chain ...
METHOD FOR PRODUCING A GRAFTED RUBBER AND TIRE COMPRISING THE GRAFTED RUBBER
-
Page/Page column 7, (2020/01/24)
The present invention relates to a metho...
461642-78-4 Process route
-
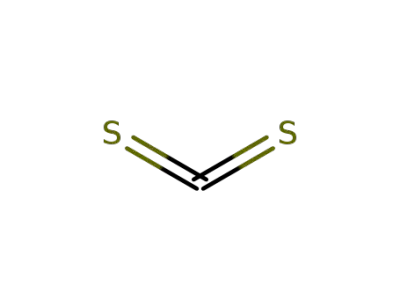
-
75-15-0,12122-00-8
carbon disulfide

-
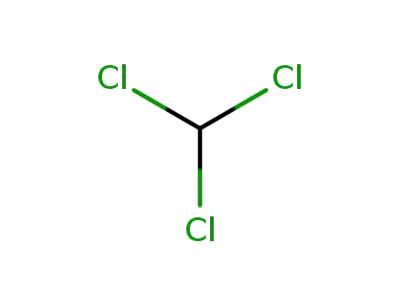
-
67-66-3,8013-54-5
chloroform

-
-
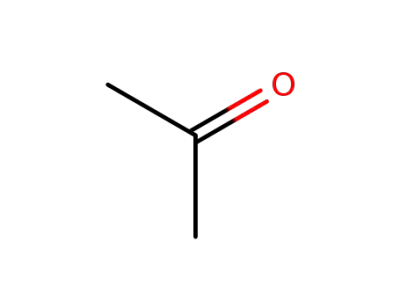
67-64-1
acetone

-

-
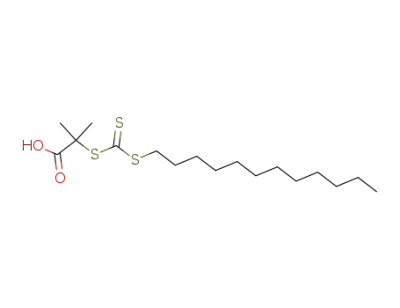
112-55-0
1-dodecylthiol

-
-
461642-78-4
2-dodecylsulfanylthiocarbonylsulfanyl-2-methyl-propionic acid
| Conditions | Yield |
|---|---|
|
carbon disulfide; acetone; 1-dodecylthiol;
With
sodium hydroxide; Aliquat 336;
trimethyloctadecylammonium chloride;
In
water;
for 0.333333h;
chloroform;
With
sodium hydroxide;
In
water;
at 15 - 20 ℃;
With
hydrogenchloride;
In
water;
|
85% |
|
1-dodecylthiol;
With
Aliquat 336; sodium hydroxide;
In
water; acetone;
at 10 ℃;
for 0.666667h;
Inert atmosphere;
carbon disulfide; acetone;
In
water;
at 10 ℃;
for 0.5h;
Inert atmosphere;
chloroform;
Further stages;
|
85% |
|
1-dodecylthiol;
With
sodium hydroxide;
In
acetone;
at 20 ℃;
for 0.5h;
carbon disulfide; acetone;
In
acetone;
at 20 ℃;
for 0.166667h;
chloroform;
With
sodium hydroxide;
at 20 - 25 ℃;
|
48% |
|
1-dodecylthiol;
With
tetrabutylammomium bromide; sodium hydroxide;
In
water; acetone;
at 10 ℃;
for 0.583333h;
Inert atmosphere;
carbon disulfide;
In
acetone;
for 0.5h;
chloroform; acetone;
Further stages;
|
|
|
1-dodecylthiol;
With
sodium hydroxide;
In
water; acetone;
at 10 ℃;
for 0.5h;
carbon disulfide;
In
water; acetone;
for 0.5h;
chloroform; acetone;
With
sodium hydroxide;
In
water;
|
55.2 g |
|
acetone; 1-dodecylthiol;
With
Aliquat 336; sodium hydroxide;
at 10 ℃;
for 0.583333h;
Inert atmosphere;
carbon disulfide;
for 0.5h;
Inert atmosphere;
chloroform;
Inert atmosphere;
|
-

-
75-15-0,12122-00-8
carbon disulfide

-
-
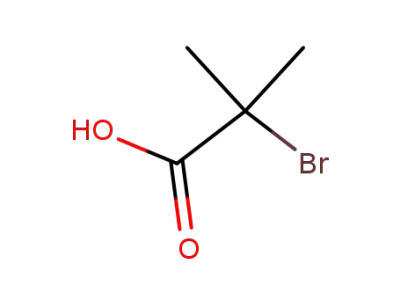
2052-01-9
2-bromo-2-methylpropionic acid

-

-
112-55-0
1-dodecylthiol

-

-
461642-78-4
2-dodecylsulfanylthiocarbonylsulfanyl-2-methyl-propionic acid
| Conditions | Yield |
|---|---|
|
carbon disulfide; 1-dodecylthiol;
With
potassium phosphate;
In
acetone;
for 0.666667h;
2-bromo-2-methylpropionic acid;
In
acetone;
at 20 ℃;
|
65% |
|
1-dodecylthiol;
With
potassium phosphate;
In
acetone;
carbon disulfide;
In
acetone;
at 0 ℃;
2-bromo-2-methylpropionic acid;
In
acetone;
at 20 ℃;
for 24h;
|
60% |
|
1-dodecylthiol;
With
sodium hydroxide;
In
water; acetone;
at 10 ℃;
for 0.166667h;
carbon disulfide;
In
water; acetone;
for 0.166667h;
2-bromo-2-methylpropionic acid;
In
water; acetone;
at 20 ℃;
for 24h;
|
47.3% |
|
1-dodecylthiol;
With
potassium phosphate;
In
acetone;
for 0.166667h;
carbon disulfide;
In
acetone;
for 0.166667h;
2-bromo-2-methylpropionic acid;
In
acetone;
at 20 ℃;
for 13h;
Inert atmosphere;
|
43% |
|
1-dodecylthiol;
With
potassium phosphate;
In
acetone;
for 0.166667h;
carbon disulfide;
In
acetone;
for 0.5h;
2-bromo-2-methylpropionic acid;
at 20 ℃;
for 14h;
|
37% |
|
carbon disulfide; 1-dodecylthiol;
With
potassium phosphate;
In
acetone;
at 0 ℃;
for 0.166667h;
2-bromo-2-methylpropionic acid;
In
acetone;
at 20 ℃;
|
35.8% |
|
carbon disulfide; 1-dodecylthiol;
With
potassium hydroxide;
In
water; acetone;
at 20 ℃;
for 3h;
Cooling with ice;
2-bromo-2-methylpropionic acid;
In
water; acetone;
|
33% |
|
1-dodecylthiol;
With
potassium phosphate;
In
acetone;
for 0.5h;
carbon disulfide;
In
acetone;
for 0.333333h;
2-bromo-2-methylpropionic acid;
In
acetone;
for 21h;
|
32.4% |
|
With
potassium phosphate;
In
acetone;
at 20 ℃;
for 20h;
|
|
|
1-dodecylthiol;
With
potassium phosphate;
In
acetone;
at 20 ℃;
carbon disulfide;
In
acetone;
at 20 ℃;
2-bromo-2-methylpropionic acid;
In
acetone;
at 20 ℃;
for 24h;
|
461642-78-4 Upstream products
-
75-15-0

carbon disulfide
-
67-66-3

chloroform
-
67-64-1

acetone
-
112-55-0

1-dodecylthiol
461642-78-4 Downstream products
-
1174764-26-1
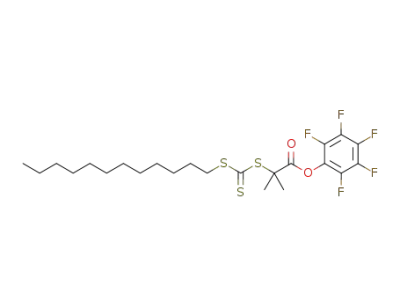
S-dodecyl-S'-(α,α-dimethylpentafluorophenyl acetate)trithiocarbonate
-
1204502-18-0
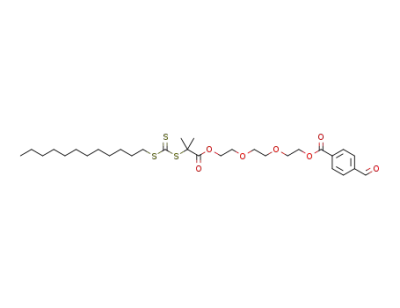
C31H48O7S3
-
1292766-77-8
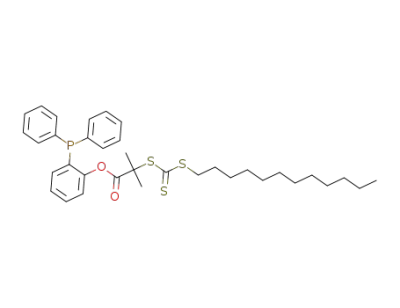
C35H45O2PS3
-
1334532-10-3
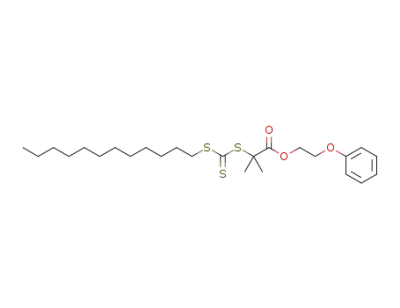
2-(dodecylthiocarbonothioylthio)-2-methylpropanoic acid 2-phenoxyethyl ester
Relevant Products
-
1-Fluoropyridinium Triflate
CAS:107263-95-6
-
2,2'-[(Thioxomethylene)Disulfanyl]Bis(2-Methylpropanoic Acid)
CAS:355120-40-0
-
Zyl 9H-Carbazole-9-Carbodithioate
CAS:137780-73-5