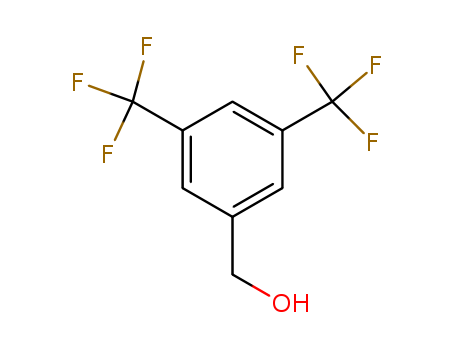
32707-89-4
- Product Name:3,5-Bis(Trifluoromethyl)Benzyl Alcohol
- MF:C9H6F6O
- Purity:99%
- Molecular Weight:244.136
Product Details
High quality purity >99% 3,5-Bis(Trifluoromethyl)Benzyl Alcohol 32707-89-4 for sale
- Molecular Formula:C9H6F6O
- Molecular Weight:244.136
- Appearance/Colour:white to light yellow crystal powder
- Vapor Pressure:0.823mmHg at 25°C
- Melting Point:53-56 °C(lit.)
- Refractive Index:1.415
- Boiling Point:173.9 °C at 760 mmHg
- PKA:13.92±0.10(Predicted)
- Flash Point:97.8 °C
- PSA:20.23000
- Density:1.433 g/cm3
- LogP:3.21650
3,5-Bis(trifluoromethyl)benzyl alcohol(Cas 32707-89-4) Usage
InChI:InChI=1/C9H6F6O/c10-8(11,12)6-1-5(4-16)2-7(3-6)9(13,14)15/h1-3,16H,4H2
32707-89-4 Relevant articles
Preparation process of high-purity 3,5-bis(trifluoromethyl)benzyl alcohol
-
Paragraph 0042-0048, (2021/08/07)
The invention discloses a preparation me...
Tunable Ligand Effects on Ruthenium Catalyst Activity for Selectively Preparing Imines or Amides by Dehydrogenative Coupling Reactions of Alcohols and Amines
Higuchi, Takafumi,Tagawa, Risa,Iimuro, Atsuhiro,Akiyama, Shoko,Nagae, Haruki,Mashima, Kazushi
supporting information, p. 12795 - 12804 (2017/09/06)
Selective dehydrogenative synthesis of i...
Palladium-catalyzed arylation of aldehydes with bromo-substituted 1,3-diaryl-imidazoline carbene ligand
Yamamoto, Tetsuya,Furusawa, Takuma,Zhumagazin, Azamat,Yamakawa, Tetsu,Oe, Yohei,Ohta, Tetsuo
, p. 19 - 26 (2015/02/19)
The combination of 0 valent palladium pr...
Palladium-catalyzed hydroxymethylation of aryl-and heteroarylboronic acids using aqueous formaldehyde
Yamamoto, Tetsuya,Zhumagazin, Azamat,Furusawa, Takuma,Tanaka, Ryoji,Yamakawa, Tetsu,Oe, Yohei,Ohtab, Tetsuo
supporting information, p. 3525 - 3529 (2015/01/09)
Cyclometallated NHC palladium complexes ...
32707-89-4 Process route
-
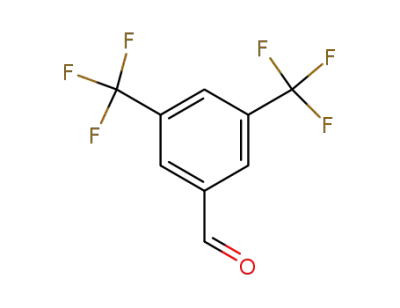
-
401-95-6
3,5-Bis(trifluoromethyl)benzaldehyde

-
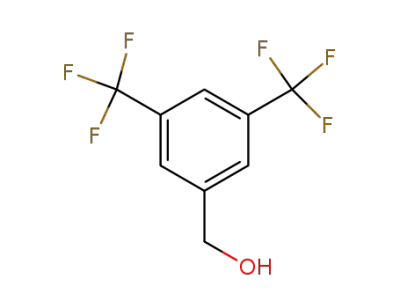
-
32707-89-4
3,5-bis(trifluoromethyl)benzenemethanol
| Conditions | Yield |
|---|---|
|
With
methanol; sodium tetrahydroborate;
at 20 - 30 ℃;
Temperature;
Reagent/catalyst;
|
96% |
|
With
hydrogen;
nickel;
In
toluene;
at 50 ℃;
for 7.5h;
under 22502.3 Torr;
|
82.5% |
|
With
hydrogen;
aluminum nickel;
In
toluene;
|
82.5% |
-
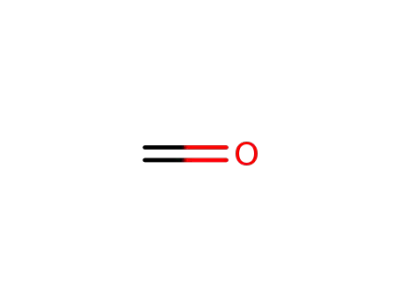
-
50-00-0,30525-89-4,61233-19-0
formaldehyd

-
-
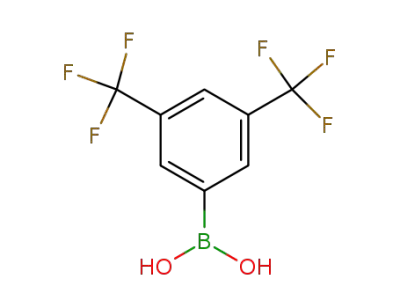
73852-19-4
3,5-bis-trifluromethylphenylboronic acid

-

-
32707-89-4
3,5-bis(trifluoromethyl)benzenemethanol
| Conditions | Yield |
|---|---|
|
With
bis(η3-allyl-μ-chloropalladium(II)); 1-(2-bromophenyl)-3-(2,6-diisopropylphenyl)-4,5-dihydroimidazolinium chloride;
In
tetrahydrofuran; water;
at 100 ℃;
for 2h;
Inert atmosphere;
Sealed tube;
|
85% |
|
With
di-μ-chloro-bis{2-[3-(2,6-diisopropylphenyl)imidazolin-2-ylidene]-(3-phenylthio)phenyl-κ2C,C’}dipalladium(II); caesium carbonate;
In
tetrahydrofuran; water;
at 70 ℃;
for 2h;
Inert atmosphere;
Sealed tube;
|
76% |
32707-89-4 Upstream products
-
725-89-3
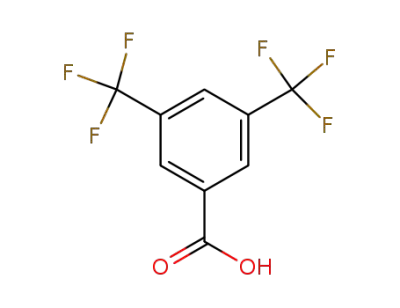
3,5-bistrifluoromethylbenzoic acid
-
401-95-6

3,5-Bis(trifluoromethyl)benzaldehyde
-
557-20-0
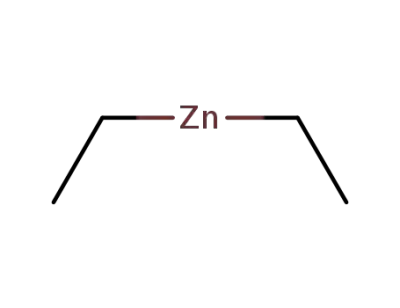
diethylzinc
-
50-00-0
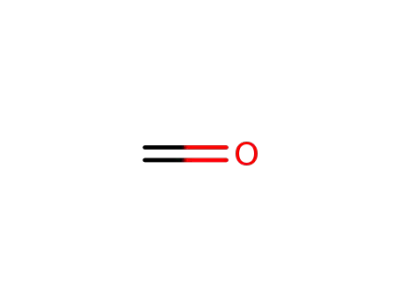
formaldehyd
32707-89-4 Downstream products
-
401-95-6

3,5-Bis(trifluoromethyl)benzaldehyde
-
159707-06-9
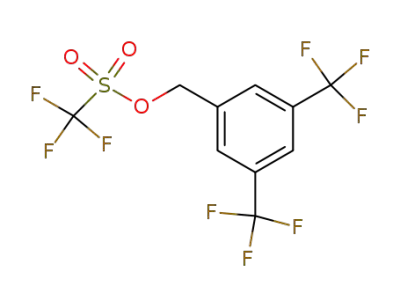
3,5-bis-(trifluoromethyl)benzyl trifluoromethanesulfonate
-
374819-27-9
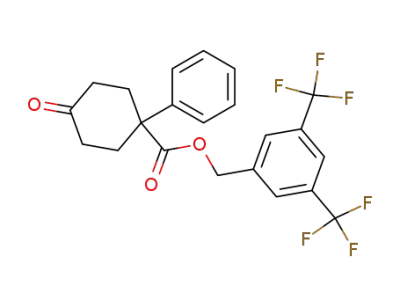
3,5-bis(trifluoromethyl)phenylmethyl 4-oxo-1-phenylcyclohexanecarboxylate
-
345579-46-6
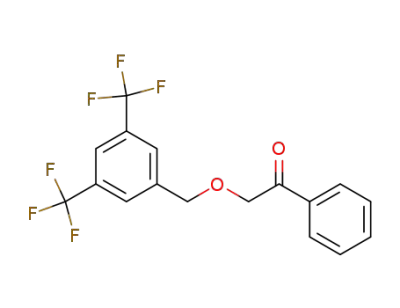
2-(3,5-bis(trifluoromethyl)benzyloxy)-1-phenylethanone
Relevant Products
-
3,5-Bis(Trifluoromethyl)Benzoyl Chloride
CAS:785-56-8
-
1,8-Diiodoperfluorooctane
CAS:335-70-6
-
2,3,3',4'-Biphenyl Tetracarboxylic Acid Dianhydride
CAS:36978-41-3