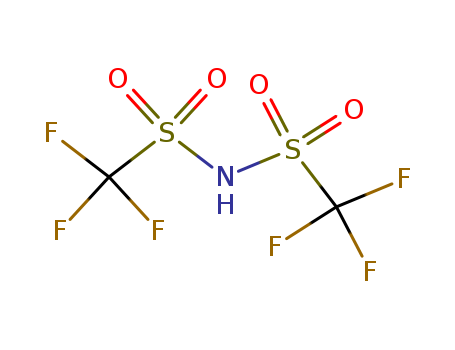
82113-65-3
- Product Name:Trifluoromethanesulfonimide
- MF:C2HF6NO4S2
- Purity:99%
- Molecular Weight:281.157
Product Details
Cost-effective and customizable Trifluoromethanesulfonimide 82113-65-3 supplier
- Molecular Formula:C2HF6NO4S2
- Molecular Weight:281.157
- Appearance/Colour:light yellow to brown liquid
- Vapor Pressure:0.274mmHg at 25°C
- Melting Point:52-56 °C
- Refractive Index:1.523
- Boiling Point:190.5 °C at 760 mmHg
- PKA:-10.42±0.40(Predicted)
- Flash Point:69 °C
- PSA:97.07000
- Density:1.936 g/cm3
- LogP:2.82770
TRIFLUOROMETHANESULFONIMIDE(Cas 82113-65-3) Usage
|
General Description |
Bis(trifluoromethane)sulfonimide (TFSI, Tf2N) is utilized as anion species to form 1-ethyl-3-methylimidazolium molten salts. It undergoes acid-base complexation with rod-like poly(2,5-pyridine) to afford highly-ordered lamellar self-assemblies in the hydrated films. |
InChI:InChI=1/C8H7F3OS/c9-8(10,11)13-7-3-1-2-6(4-7)5-12/h1-4,12H,5H2
82113-65-3 Relevant articles
Thermally stable bis(trifluoromethylsulfonyl)imide salts and their mixtures
Scheuermeyer, Marlene,Kusche, Matthias,Agel, Friederike,Schreiber, Patrick,Maier, Florian,Steinrück, Hans-Peter,Davis, James H.,Heym, Florian,Jess, Andreas,Wasserscheid, Peter
, p. 7157 - 7161 (2016)
We show that both tetraphenylphosphonium...
N-FLUORO-BIS(TRIFLUOROMETHANESULFONYL)IMIDE AN IMPROVED SYNTHESIS
Desmarteau, Darryl D.,Witz, Michael
, p. 7 - 12 (1991)
An improved synthesis of (CF3SO2)2NH and...
A one-pot synthesis of a ternary nanocomposite based on mesoporous silica, polyaniline and silver
Rosa, Ana Claudia De Abreu,Correa, Cintia Marques,Faez, Roselena,Bizeto, Marcos Augusto,Camilo, Fernanda Ferraz
, p. 26142 - 26148 (2013)
The research on hybrid materials compose...
PERFLUOROALKYL SULFONAMIDE AND METHOD FOR PRODUCING THE SAME
-
Paragraph 0070-0074, (2018/06/05)
PROBLEM TO BE SOLVED: To simply provide ...
Silylium-Catalyzed Carbon–Carbon Coupling of Alkynylsilanes with (2-Bromo-1-methoxyethyl)arenes: Alternative Approaches
Rubial, Belén,Ballesteros, Alfredo,González, José M.
supporting information, p. 6194 - 6198 (2018/07/31)
The catalytic activation of alkynylsilan...
Preparation method of LiN(CF3SO2)2 salt
-
Paragraph 0044; 0046; 0049; 0052; 0054, (2018/02/04)
The invention discloses a preparation me...
METHOD FOR PRODUCING TRIFLUOROMETHANESULFONYL IMIDE OR ITS SALT
-
Paragraph 0154; 0155, (2017/02/28)
The present invention relates to trifluo...
82113-65-3 Process route
-

-
41804-81-3
(CH3)3CN(SO2CF3)2

-
-
82113-65-3
bis(trifluoromethanesulfonyl)amide

-
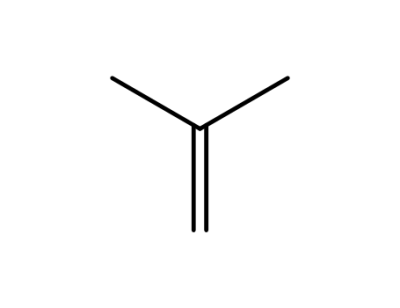
-
115-11-7,15220-85-6
isobutene
| Conditions | Yield |
|---|---|
|
decompn. with quantitative elimination of (CH3)2CH2;
|
>99 |
|
decompn. with quantitative elimination of (CH3)2CH2;
|
>99 |
-
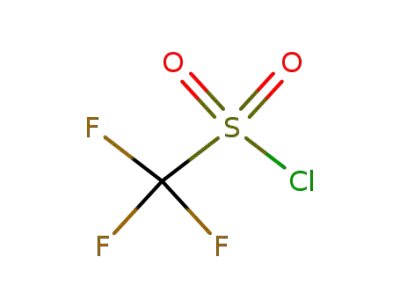
-
421-83-0
trifluoromethane sulfonyl chloride

-
-
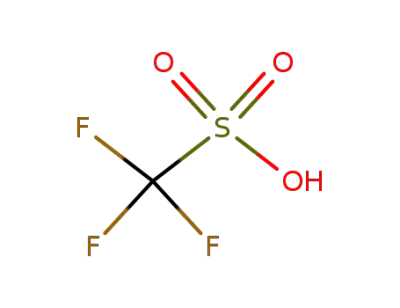
1493-13-6
trifluorormethanesulfonic acid

-

-
82113-65-3
bis(trifluoromethanesulfonyl)amide
| Conditions | Yield |
|---|---|
|
trifluoromethane sulfonyl chloride;
With
dmap; ammonia; N-ethyl-N,N-diisopropylamine;
In
dichloromethane;
at 0 - 20 ℃;
With
dihydrogen peroxide;
In
water;
at 80 ℃;
|
82113-65-3 Upstream products
-
91742-21-1
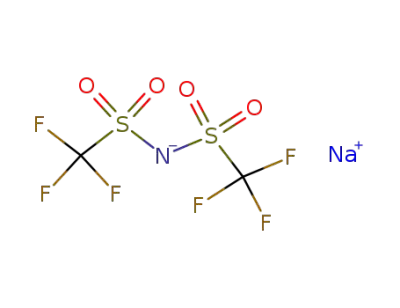
sodium bis(trifluoromethanesulfonyl)imide
-
91742-20-0
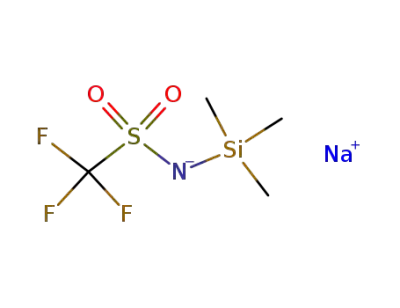
N-(trimethylsilyl)trifluoromethanesulfonamide N-sodium salt
-
90076-65-6
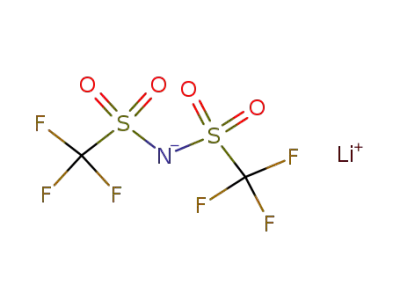
bis(trifluoromethane)sulfonimide lithium
-
41804-81-3

(CH3)3CN(SO2CF3)2
82113-65-3 Downstream products
-
149108-74-7
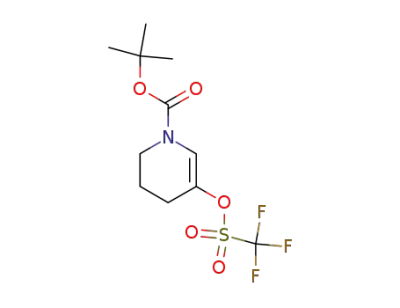
5-trifluoromethanesulfonyloxy-3,4-dihydro-2H-pyridine-1-carboxylic acid tert-butyl ester
-
180691-65-0
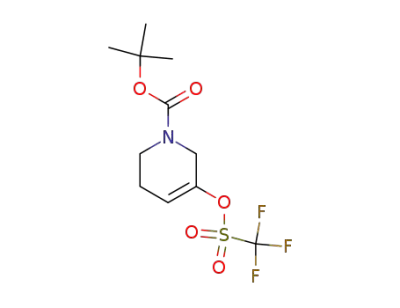
5-trifluoromethanesulfonyloxy-3,6-dihydro-2H-pyridine-1-carboxylic acid tert-butyl ester
-
187737-37-7
propene
-
82113-66-4
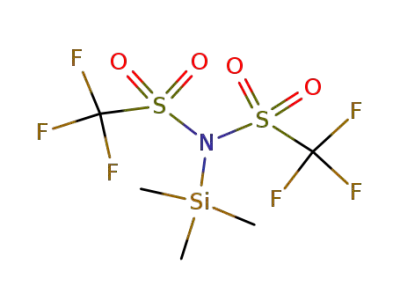
trimethylsilyl bis(trifluoromethanesulfonyl)imide
Relevant Products
-
1-Fluoropyridinium Triflate
CAS:107263-95-6
-
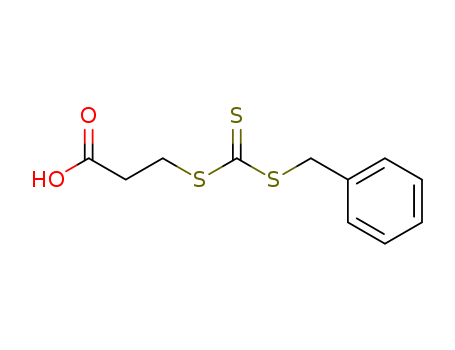
Propanoic Acid, 3-[[[(Phenylmethyl)Thio]Thioxomethyl]Thio]-
CAS:497931-76-7
-
3-Pyridinamine, 6,6′-[1,2-Ethanediylbis(Oxy)]Bis-
CAS:2243795-22-2