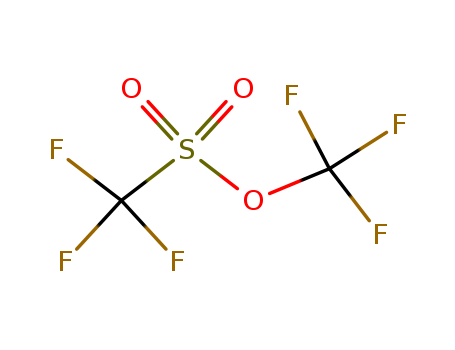
3582-05-6
- Product Name:Trifluoromethanesulfonic Acid Trifluoromethyl Ester
- MF:C2F6O3S
- Purity:99%
- Molecular Weight:218.077
Product Details
Cost-effective and customizable Trifluoromethanesulfonic Acid Trifluoromethyl Ester 3582-05-6 supplier
- Molecular Formula:C2F6O3S
- Molecular Weight:218.077
- Vapor Pressure:359mmHg at 25°C
- Melting Point:-108,2 °C
- Refractive Index:1.297
- Boiling Point:45.6 °C at 760 mmHg
- PSA:51.75000
- Density:1.791 g/cm3
- LogP:2.45320
TRIFLUOROMETHANESULFONIC ACID TRIFLUOROMETHYL ESTER(Cas 3582-05-6) Usage
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 52, p. 4147, 1987 DOI: 10.1021/jo00228a001 |
InChI:InChI=1/C2F6O3S/c3-1(4,5)11-12(9,10)2(6,7)8
3582-05-6 Relevant articles
THERMOLYSE DES ANHYDRIDES PERFLUOROALCANESULFONIQUES. MECANISME ET APPLICATION A LA SYNTHESE DES ESTERS RFSO3RF.
Oudrhiri-Hassani, M.,Brunel, D.,Germain, A.,Commeyras, A.
, p. 219 - 232 (1984)
Perfluoroalkanesulfonic anhydrides FSO2)...
Gas-phase structure and vibrational properties of trifluoromethyl trifluoromethanesulfonate, CF3SO2OCF3
Tuttolomondo, Maria E.,Arganaraz, Pablo E.,Varetti, Eduardo L.,Hayes, Stuart A.,Wann, Derek A.,Robertson, Heather E.,Rankin, David W. H.,Altabef, Aida Ben
, p. 1381 - 1389 (2007)
Trifluoromethyl trifluoromethanesulfonat...
Palladium(II)-Catalyzed Enantioselective Aminotrifluoromethoxylation of Unactivated Alkenes using CsOCF3 as a Trifluoromethoxide Source
Chen, Chaohuang,Pflüger, Philipp Miro,Chen, Pinhong,Liu, Guosheng
, p. 2392 - 2396 (2019)
Asymmetric PdII-catalyzed intramolecular...
THERMAL STABILITY OF PERFLUOROALKANESULFONIC ACIDS AND THEIR ANHYDRIDES. NEW AND EASY APPROACH TO RFSO2ORF ESTERS.
Hassani, M. Oudrhiri,Germain, A.,Brunel, D.,Commeyras, A.
, p. 65 - 68 (1981)
Perfluoroalkanesulfonic anhydrides (RFSO...
Tertiary-Amine-Initiated Synthesis of Acyl Fluorides from Carboxylic Acids and CF3SO2OCF3
Song, Hai-Xia,Tian, Ze-Yu,Xiao, Ji-Chang,Zhang, Cheng-Pan
supporting information, p. 16261 - 16265 (2020/12/01)
A convenient method for deoxyfluorinatio...
Silver-Mediated Trifluoromethoxylation of (Hetero)aryldiazonium Tetrafluoroborates
Yang, Yu-Ming,Yao, Jian-Fei,Yan, Wei,Luo, Zhuangzhu,Tang, Zhen-Yu
supporting information, p. 8003 - 8007 (2019/10/11)
Here we report a silver-mediated trifluo...
Synthesis of heteroaromatic trifluoromethyl ethers with trifluoromethyl triflate as the source of the trifluoromethoxy group
Zhang, Qing-Wei,Hartwig, John F.
supporting information, p. 10124 - 10127 (2018/09/18)
A series of nitrogen-heterocycles have b...
3582-05-6 Process route
-
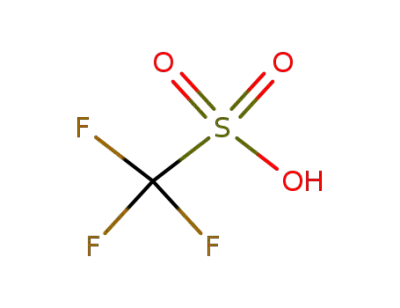
-
1493-13-6
trifluorormethanesulfonic acid

-

-
75-05-8,26809-02-9
acetonitrile

-
-
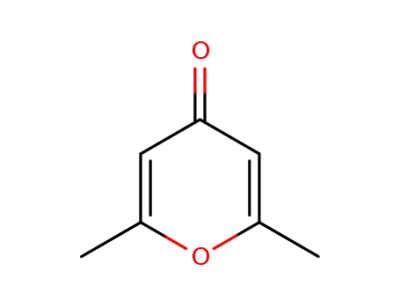
1004-36-0
2,6-dimethylpyrone

-
-
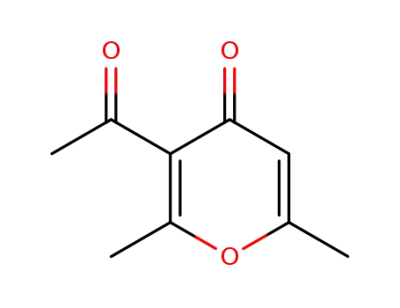
7521-38-2
3-acetyl-2,6-dimethyl-4H-pyran-4-one

-
-
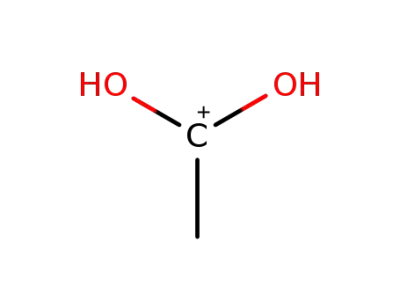
31111-45-2
C2H7N2(1+)

-
-
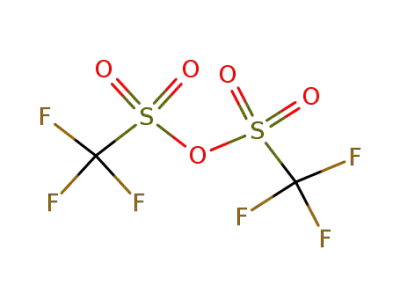
358-23-6
trifluoromethylsulfonic anhydride

-
-
17104-45-9,18639-92-4
acetacidium

-
-
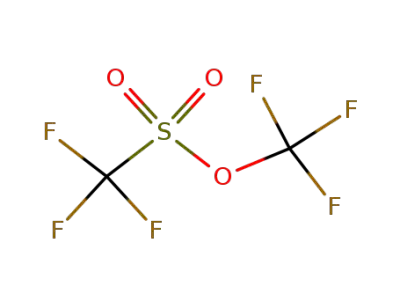
3582-05-6
trifluoromethyl trifluoromethanesulfonate

-
-
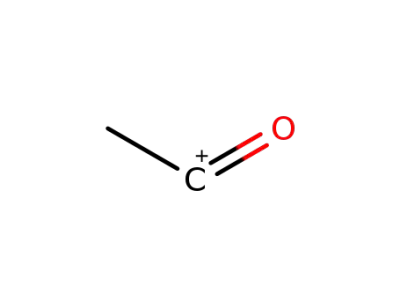
15762-07-9
oxoethylium

-
-
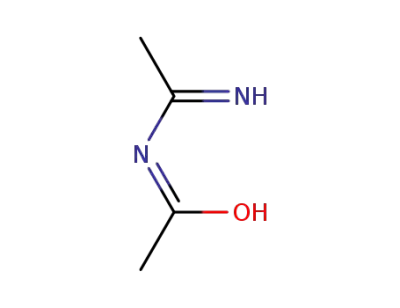
1361550-85-7,5699-42-3
N-acetylacetamidine

-
-
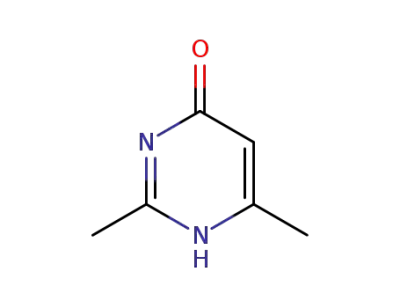
6622-92-0
2,4-dimethyl-1,6-dihydro-6-pyrimidone
| Conditions | Yield |
|---|---|
|
In
dichloromethane-d2;
at 74 ℃;
Cooling;
|
-

-
1493-13-6
trifluorormethanesulfonic acid

-
-
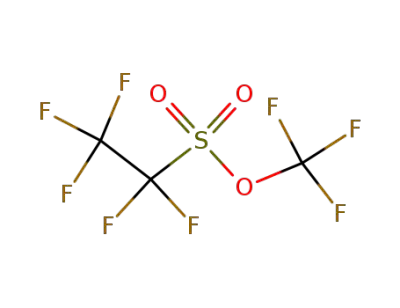
77927-85-6
pentafluoroethanesulfonate de trifluoromethyle

-
-
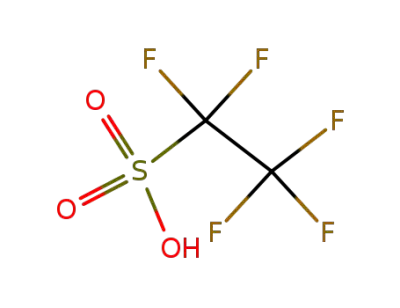
354-88-1
perfluoroethanesulfonic acid

-

-
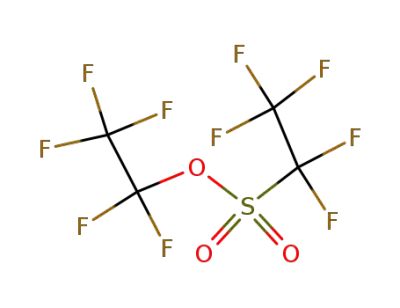
3582-05-6
trifluoromethyl trifluoromethanesulfonate

-
-
77927-84-5
pentafluoroethyl pentafluoroethanesulfonate
| Conditions | Yield |
|---|---|
|
at 190 ℃;
for 40h;
Product distribution;
|
3582-05-6 Upstream products
-
1493-13-6

trifluorormethanesulfonic acid
-
116-15-4
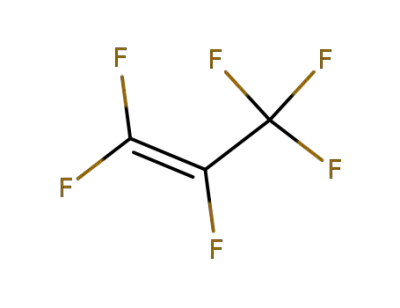
perfluoropropylene
-
70142-16-4
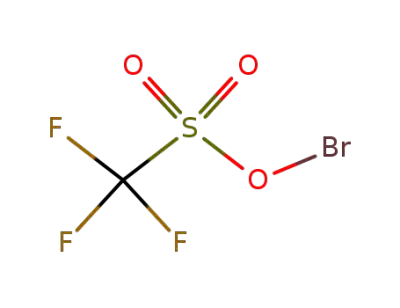
Bromine(I) trifluoromethanesulfonate
-
75-71-8
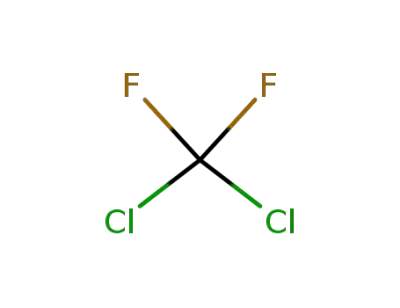
Dichlorodifluoromethane
3582-05-6 Downstream products
-
335-05-7
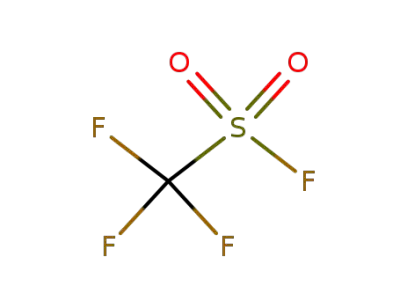
Trifluoromethanesulfonyl fluoride
-
1493-13-6

trifluorormethanesulfonic acid
-
77927-85-6

pentafluoroethanesulfonate de trifluoromethyle
-
77927-84-5

pentafluoroethyl pentafluoroethanesulfonate
Relevant Products
-
Nonafluorobutanesulfonic Anhydride 97
CAS:36913-91-4
-
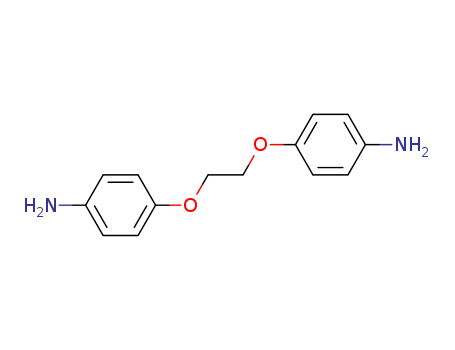
1,2-Bis(4-Aminophenoxy)Ethane
CAS:6052-10-4
-
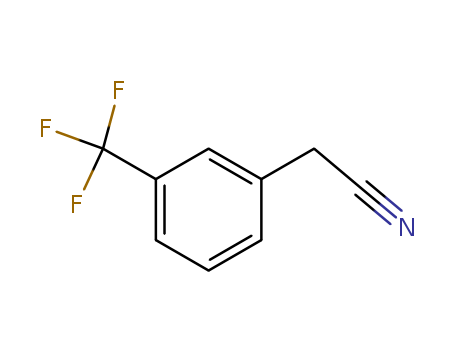
3-Trifluoromethylbenzylcyanide
CAS:2338-76-3