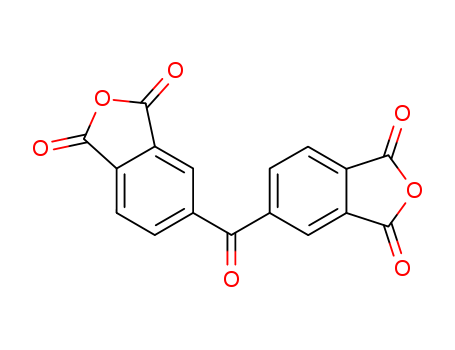
2421-28-5
- Product Name:3,3',4,4'-Diphenylketone Tetracarboxylic Acid Dianhydride
- MF:C17H6O7
- Purity:99%
- Molecular Weight:322.23
Product Details
3,3',4,4'-Diphenylketone Tetracarboxylic Acid Dianhydride 2421-28-5 with purity >99% Low price in stock
- Molecular Formula:C17H6O7
- Molecular Weight:322.23
- Appearance/Colour:white crystal powder
- Vapor Pressure:<0.1 mm Hg ( 0 °C)
- Melting Point:218-222 °C(lit.)
- Refractive Index:1.6380 (estimate)
- Boiling Point:638.8 °C at 760 mmHg
- Flash Point:286.1 °C
- PSA:103.81000
- Density:1.662 g/cm3
- LogP:1.53880
2421-28-5 Relevant articles
Development and optimization of producing 3,3′, 4,4′-benzophenonetetracarboxylic dianhydride
Yegorov, Anton Sergeyevich,Wozniak, Alyona Igorevna,Ivanov, Vitaly Sergeyevich,Averina, Elena Aleksandrovna,Zhdanovich, Olga Anatolevna
, p. 3063 - 3070 (2016)
This paper includes a series of experime...
A 3,3 the [...], 4,4 the method for preparing [...] -benzophenone tetracidic dianhydride
-
Paragraph 0024-0027, (2017/02/09)
The invention discloses a method for syn...
Bronsted base-assisted boronic acid catalysis for the dehydrative intramolecular condensation of dicarboxylic acids
Sakakura, Akira,Ohkubo, Takuro,Yamashita, Risa,Akakura, Matsujiro,Ishihara, Kazuaki
supporting information; experimental part, p. 892 - 895 (2011/05/02)
Bronsted base-assisted boronic acid cata...
METHOD FOR PRODUCING CARBOXYLIC ANHYDRIDE AND ARYLBORONIC ACID COMPOUND
-
Page/Page column 10-11, (2012/01/13)
When phthalic acid is heated in heptane ...
2421-28-5 Process route
-
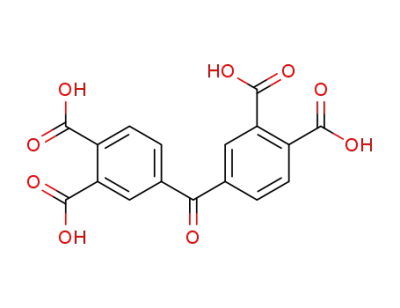
-
2479-49-4
3,3',4,4'-benzophenonetetracarboxylic acid

-
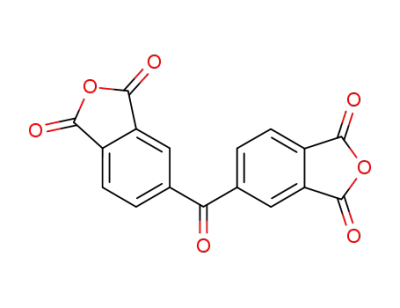
-
2421-28-5
dianhydride of benzophenone-3,4,3',4'-tetracarboxy acid
| Conditions | Yield |
|---|---|
|
With
2,6-bis[(2,2,6,6-tetramethylpiperidin-1-yl)methyl]phenylboronic acid;
In
propyl cyanide;
for 12h;
Reflux;
|
98% |
|
at 225 ℃;
for 2h;
Industrial scale;
|
73.56% |
|
In
diphenylether;
at 230 ℃;
for 2h;
Dean-Stark;
Heating;
|
70% |
-
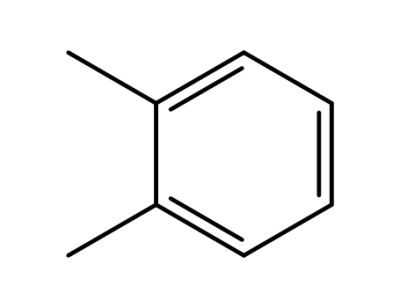
-
95-47-6
o-xylene

-

-
2421-28-5
dianhydride of benzophenone-3,4,3',4'-tetracarboxy acid
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1.1: 3 h / 0 - 2 °C / Inert atmosphere; Industrial scale
1.2: 7 h / 0 - 40 °C / Inert atmosphere; Industrial scale
2.1: potassium permanganate; hydrogenchloride / water / 3 h / 80 °C / Industrial scale
3.1: 2 h / 225 °C / Industrial scale
With
hydrogenchloride; potassium permanganate;
In
water;
|
|
|
Multi-step reaction with 4 steps
1: aluminum (III) chloride / 0 - 5 °C
2: sodium hydroxide / water / 1 h / Heating
3: potassium permanganate; water / pyridine / 85 °C
4: diphenylether / 2 h / 230 °C / Dean-Stark; Heating
With
aluminum (III) chloride; potassium permanganate; water; sodium hydroxide;
In
pyridine; diphenylether; water;
1: |Friedel-Crafts Alkylation;
|
2421-28-5 Upstream products
-
2479-49-4

3,3',4,4'-benzophenonetetracarboxylic acid
-
95-47-6

o-xylene
2421-28-5 Downstream products
-
47587-11-1
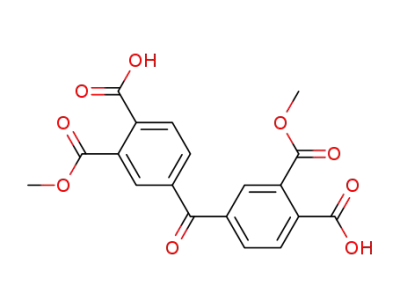
dimethyl ester of benzophenonetetracyclic acid
-
34531-33-4

3,3',4,4'-benzophenonetetracarboxylic acid ethylester
-
127324-17-8
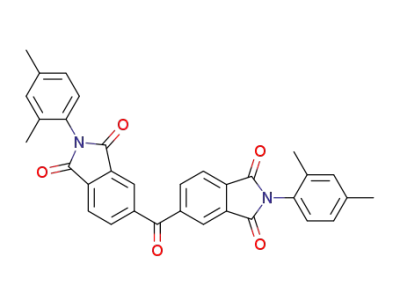
ZIN(639)C00379
-
155275-44-8
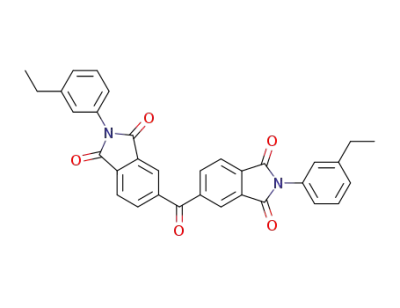
C33H24N2O5
Relevant Products
-
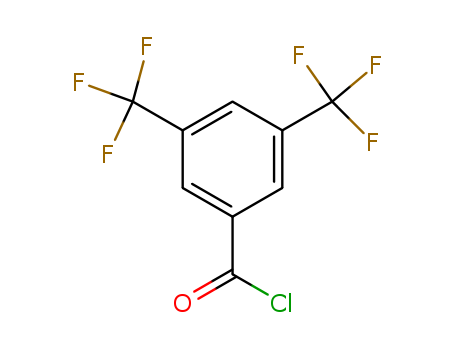
3,5-Bis(Trifluoromethyl)Benzoyl Chloride
CAS:785-56-8
-
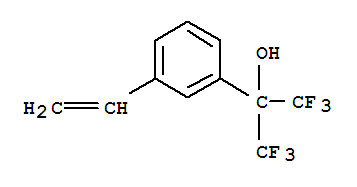
1,1,1,3,3,3-Hexafluoro-2-(4-Vinylphenyl)Propan-2-Ol
CAS:122056-08-0