21379-33-9
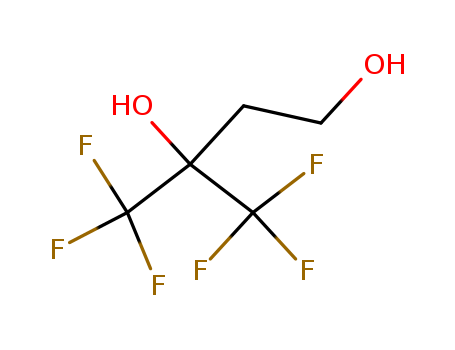
- Product Name:1,3-Butanediol, 4,4,4-Trifluoro-3-(Trifluoromethyl)-
- MF:C5H6F6O2
- Purity:99%
- Molecular Weight:212.092
Product Details
Cost-effective customized wholesale 1,3-Butanediol, 4,4,4-Trifluoro-3-(Trifluoromethyl)- 21379-33-9
- Molecular Formula:C5H6F6O2
- Molecular Weight:212.092
- Vapor Pressure:0.322mmHg at 25°C
- Refractive Index:1.3411
- Boiling Point:177.032oC at 760 mmHg
- PKA:9.35±0.29(Predicted)
- Flash Point:60.867oC
- PSA:40.46000
- Density:1.527g/cm3
- LogP:1.22450
1,3-Butanediol, 4,4,4-trifluoro-3-(trifluoromethyl)-(Cas 21379-33-9) Usage
|
General Description |
1,3-Butanediol, 4,4,4-trifluoro-3-(trifluoromethyl)- is a colorless organic compound that is commonly used as a solvent in various industries. Its chemical structure features butanediol, a four-carbon molecule with two hydroxyl groups. The addition of three fluorine atoms and a trifluoromethyl group to the butanediol molecule increases its stability and alters its physical and chemical properties. 1,3-Butanediol, 4,4,4-trifluoro-3-(trifluoromethyl)- is utilized as a solvent in the production of resins, coatings, and adhesives, as well as in the synthesis of pharmaceuticals and agricultural chemicals. Its low toxicity and high solvency make it a valuable ingredient in a wide range of industrial applications. |
InChI:InChI=1/C5H6F6O2/c6-4(7,8)3(13,1-2-12)5(9,10)11/h12-13H,1-2H2
21379-33-9 Relevant articles
Methods for Producing Fluorine-Containing Hydroxyaldehyde, Fluorine-Containing Propanediol, and Fluorine-Containing Alcohol Monomer
-
Paragraph 0213-0214, (2015/12/30)
As shown by the following reaction formu...
PROCESS FOR PREPARING FLUORINATED DIOLS
-
Page/Page column 7, (2012/09/10)
A process for preparing a fluorinated di...
THE REACTION OF SOME 3- AND 4-FLUOROOXETANES WITH ACIDS
Tarrant, Paul,Bull, R. N.
, p. 201 - 216 (2007/10/02)
Several oxetanes prepared from hexafluor...
21379-33-9 Process route
-
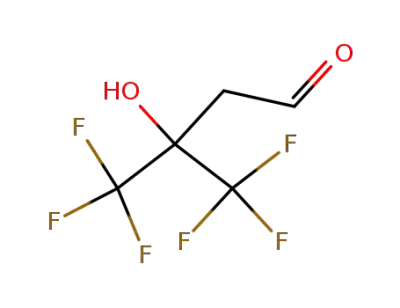
-
21379-32-8
4,4,4-Trifluor-3-hydroxy-3-(trifluormethyl)butanal

-
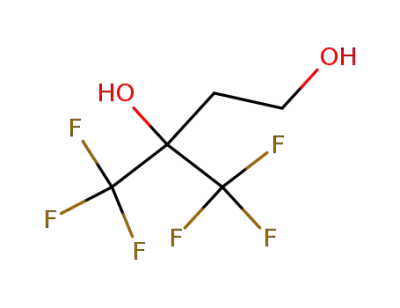
-
21379-33-9
4,4,4-trifluoro-3-(trifluoromethyl)butane-1,3-diol
| Conditions | Yield |
|---|---|
|
With
tert-butyl methyl ether; hydrogen;
palladium on activated charcoal;
at 170 ℃;
under 517.162 Torr;
|
99% |
|
With
5% active carbon-supported ruthenium; hydrogen;
In
di-isopropyl ether;
at 70 ℃;
under 13501.4 Torr;
Autoclave;
|
-
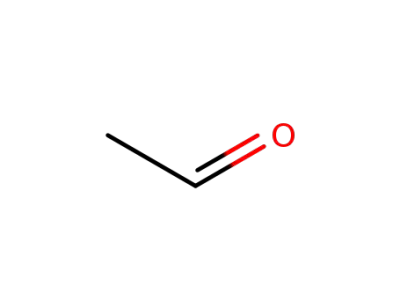
-
75-07-0,9002-91-9
acetaldehyde

-

-
684-16-2
Hexafluoroacetone

-

-
21379-33-9
4,4,4-trifluoro-3-(trifluoromethyl)butane-1,3-diol
| Conditions | Yield |
|---|---|
|
acetaldehyde; Hexafluoroacetone;
With
cesium fluoride;
at -78 - 20 ℃;
for 5h;
Autoclave;
Inert atmosphere;
With
lithium aluminium tetrahydride;
In
diethyl ether;
Cooling with ice;
|
50 %Spectr. |
21379-33-9 Upstream products
-
3440-93-5
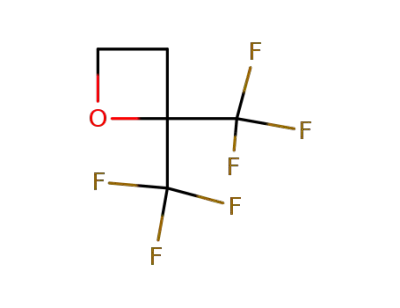
2,2-bis(trifluoromethyl)oxetane
-
21379-32-8

4,4,4-Trifluor-3-hydroxy-3-(trifluormethyl)butanal
-
75-07-0

acetaldehyde
-
684-16-2

Hexafluoroacetone
21379-33-9 Downstream products
-
1604804-96-7
C25H21F6O4S(1+)*C13H17F2O5S(1-)
Relevant Products
-
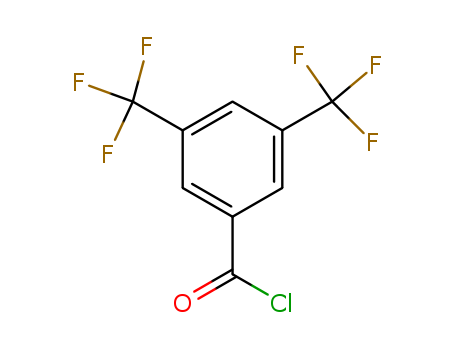
3,5-Bis(Trifluoromethyl)Benzoyl Chloride
CAS:785-56-8